.button {
float: left;
width: 100%;
padding: 10px 15px;
margin: 0 10px 20px 0;
color: #fff;
font-size: 14px;
text-transform: uppercase;
text-align: center;
border-radius: 3px;
background: #c4c5c5;
}
.button:hover {
color: #fff;
}
.button.blue {
background: #7ea7cc;
}
.button.blue:hover {
background: #9fbed8;
}
.button.green {
background: #6e8428;
}
.button.green:hover {
background: #93a261;
}
.three-column {
float: left;
width: 32%;
margin: 0 2% 40px 0;
}
.three-column.last {
margin-right: 0;
}
.anchor-nav .btn {
padding: 5px 20px;
margin-right: 10px;
}
.anchor-nav .btn.active {
background: #446b8f;
}
@media screen and ( max-width: 720px ) {
.three-column {
width: 100%;
margin-left: 0;
margin-right: 0;
}
.three-column.buttons {
margin-bottom: 20px;
}
}
Clinical evidence
Elevo®’s delivery tool allows the placement of bi-directional, barbed resorbable suture implants that provide lift to the soft palate. As the suture implants are resorbed over time, the soft palate is stiffened. Due to this lifting and stiffening action created in the soft palate, patients experienced a 30.3% reduction in sVAS at 180 days post-procedure.1
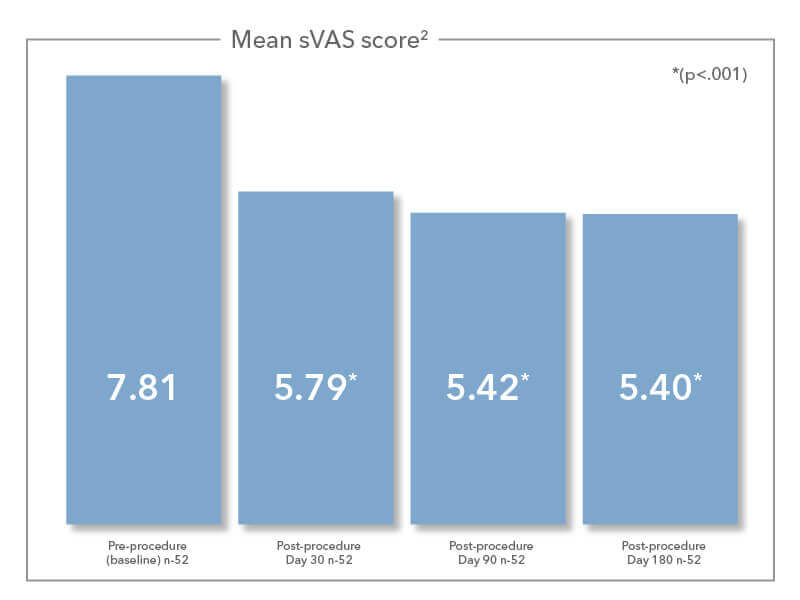
The following data are sleep outcome measurements resulting from Elevo’s 510(K) clinical trial, Snoring Intervention via Elevoplasty® in a Non-surgical Clinical Environment (S.I.LE.N.C.E.). This data were presented by Dr. Boyd Gillespie at the 2018 American Academy of Otolaryngology-Head and Neck Surgery Annual Meeting.
Epworth Sleepiness Scale
The mean score decreased from 6.63 at baseline to 4.63 at 180 days post-procedure (p<.01).
Pittsburgh Sleep Quality Index
The mean score decreased from 7.04 at baseline to 5.51 at 180 days post-procedure (p<.001).
SNAP home sleep test
The snoring loudness ratio measurements decreased, though this was not deemed statistically significant.
Learn more about the S.I.LE.N.C.E clinical trial.
- Elevo kit snoring intervention device. Premarket notification 510(k) K181107.
- Gillespie MB. Palatal foreshortening and stiffening in the treatment of primary snoring. Poster presented: The American Academy of Otolaryngology-Head and Neck Surgery Annual Meeting; October 27-30, 2018; Atlanta, GA.
