
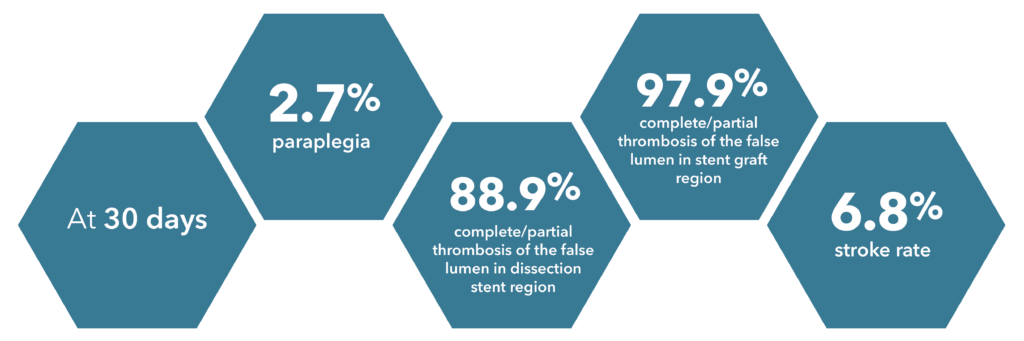
Source: Summary of Clinical Data for IFU 441-01EN (Zenith Dissection Endovascular System).
73 patients were included in the Global Clinical Study.
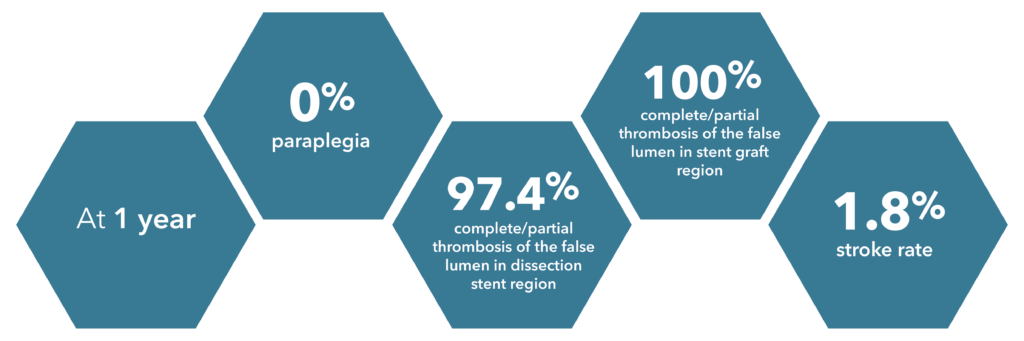
Source: Summary of Clinical Data for IFU 441-01EN (Zenith Dissection Endovascular System).
73 patients were included in the Global Clinical Study.
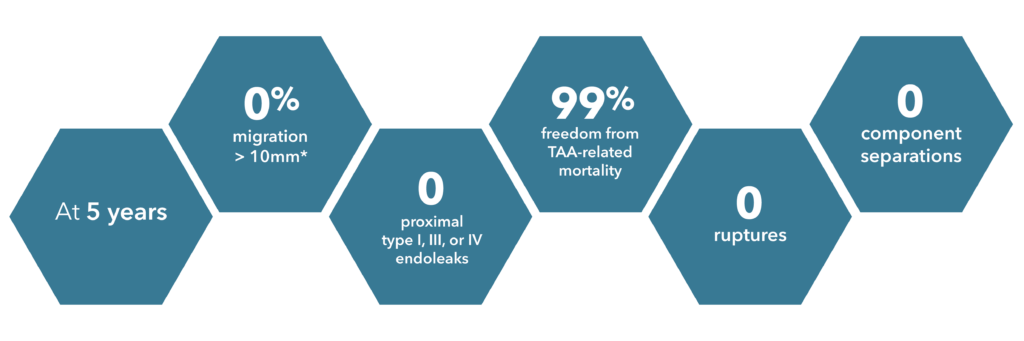
*Based upon new presentation at 5 years. (3 patients had cranial migration of the distal end, first noted at 2 years).
Source: Zenith Alpha Thoracic 2017-2018 clinical update. 110 patients were included in the Global Clinical Study.
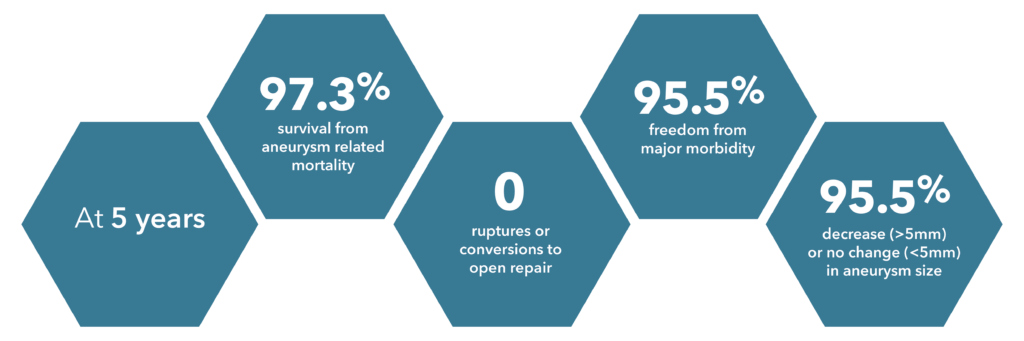
*Source: Zenith Fenestrated 2019 clinical update
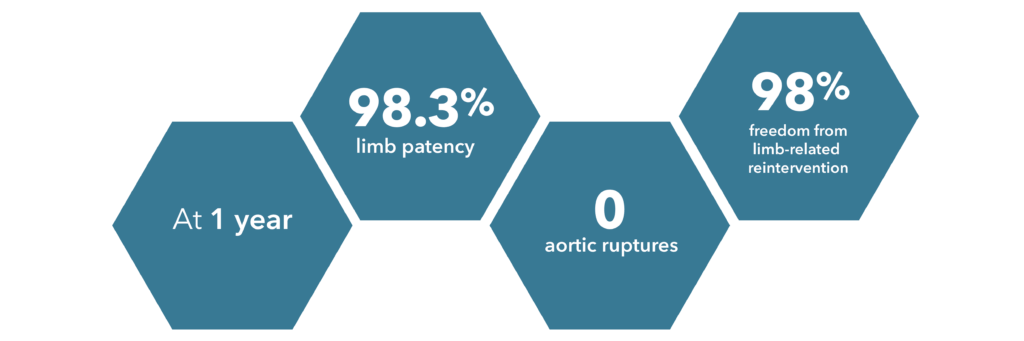
*Source: Lindsay et al., 2020
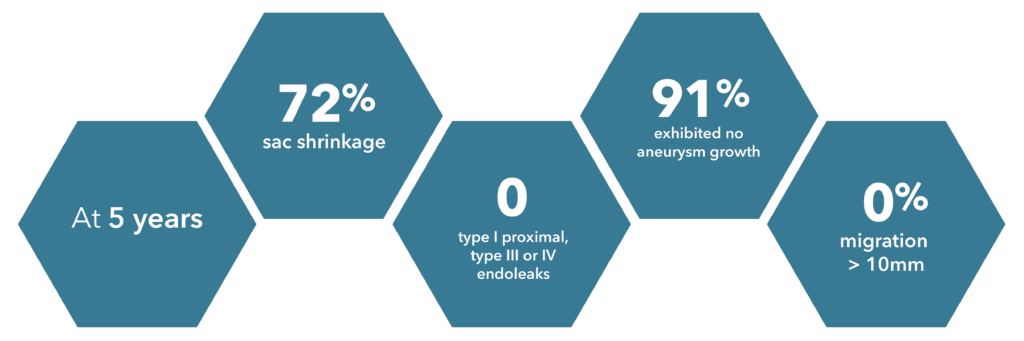
*Source: Zenith Flex 2012 clinical update
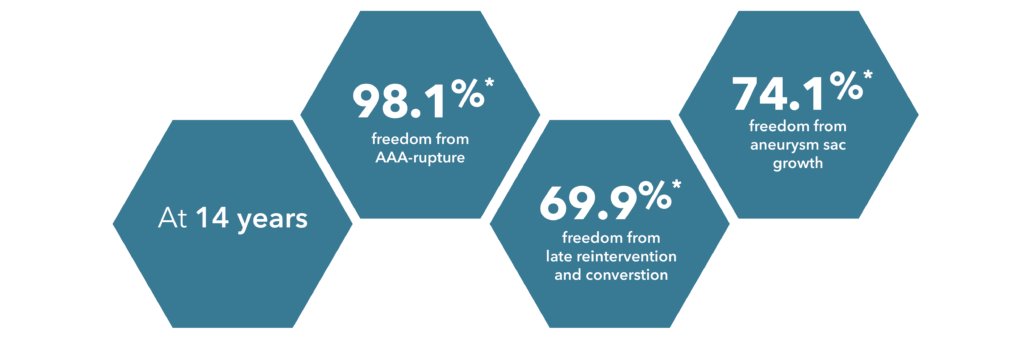
Source: Verzini et al., 2017
*These are approximate values based on Verzini et al.

*Source: Lindsay et al., 2020