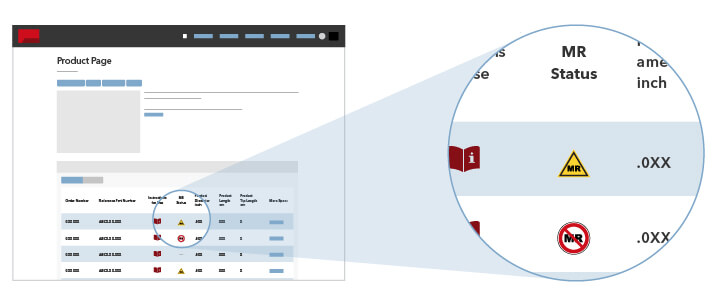
Please refer to product labeling or contact Cook Customer Support for specific information about the product’s MR status.
Find Basic Information for All Cook Products
Cook Medical adheres to the MR (magnetic resonance) safety terminology defined in ASTM International standard practice F2503-13.
Where available, MR safety information can be found on each product specification page on the Cook Medical website.
The three categories of MR safety marking are: MR Safe, MR Conditional, and MR Unsafe. In this system, “MR Safe” and “MR Unsafe” are the two extremes. For a device with an “MR Conditional” marking, the item is demonstrated to be safe under defined conditions in the MR environment. The ASTM International document provides corresponding icons that are compact and easily recognized. The terminology and icon structures are intended for use on items that may be brought into or near the MR environment and are intended to help clarify matters related to biomedical implants and devices to ensure the safe use of MRI technology.
![]() Symbol for MR Safe
Symbol for MR Safe
This symbol means an item poses no known hazards resulting from exposure to any MR environment. MR Safe items are composed of materials that are electrically nonconductive, nonmetallic, and nonmagnetic.
![]() Symbol for MR Conditional
Symbol for MR Conditional
This symbol means an item has demonstrated safety in the MR environment within defined conditions.
![]() Symbol for MR Unsafe
Symbol for MR Unsafe
This symbol means an item poses unacceptable risks to the patient, medical staff, or other persons within the MR environment.
Stainless Steel Embolization Coils
For patients implanted with stainless steel coils, recent testing shows that these coils are MR Conditional.
Nonclinical testing has demonstrated that the standard embolization coil (Stainless Steel) is MR Conditional according to ASTM F2503. A patient with this coil may be scanned safely anytime after placement under the following conditions.
- Static magnetic field of 3.0 tesla or less
- Maximum spatial magnetic gradient of 1,600 gauss/cm or less
- Maximum MR system reported, whole-body-averaged specific absorption rate (SAR) of 2.0 W/kg normal operating mode for 15 minutes of scanning or less (i.e., per scanning sequence)
Static Magnetic Field
The static magnetic field for comparison to the above limits is the static magnetic field that is pertinent to the patient (i.e., outside of scanner covering, accessible to a patient or individual).
MRI-Related Heating
In nonclinical testing, the standard embolization coil (stainless steel) produced a maximum temperature rise of 1.8 °C during 15 minutes of MR imaging (i.e., for one scanning sequence) performed in a MR 3 tesla system (General Electric Excite, Software 14X.M5) at an MR system reported whole-body-averaged SAR of 2.9 W/kg (associated with a calorimetry measured whole-body-averaged value of 2.7 W/kg).
Image Artifact
MR image quality may be compromised if the area of interest is within approximately 75 mm of the position of the standard embolization coil (stainless steel) as found during nonclinical testing using T1-weighted, spin echo and gradient echo pulse sequence in a 3.0 tesla MR system (Excite, General Electric Healthcare, Milwaukee, WI). Therefore, it may be necessary to optimize MR imaging parameters for the presence of this coil.
For U.S. Patients Only
Cook recommends that the patient register the MR conditions disclosed in this IFU with the MedicAlert Foundation. The MedicAlert Foundation can be contacted in the following manners:
MedicAlert Foundation International
2323 Colorado Avenue
Turlock, CA 95382
- Phone: 888.633.4298 (toll free)
- Alternate Phone: 209.668.3333 (from outside the U.S.)
We make every effort to keep all of our catalog items in stock. We have thousands of products, though, so we can’t guarantee that all of them will be in stock. If you have any questions about the availability of a certain product, just contact us.
Cook Medical products are delivered subject to the Limited Non-Transferrable Warranty found in item five of the North American Terms and Conditions of Sale. This warranty is exclusive and in place of all other written, oral, or implied warranties (including any warranties of merchantability or fitness for purpose). No representative of the company can offer terms different than the posted terms.
Cook reserves the right to change or discontinue any product without notice. If a product is discontinued, we can recommend alternatives.
Most Cook products have an expiration date on the label to ensure product integrity and sterility. We can’t verify an unlimited shelf life through testing, so we usually put some limit on the life of our products. Please be aware that storage conditions may affect shelf life.
Cook products should be stored in a dark, cool, dry place. Light can degrade some plastics, so don’t expose products to light for extended periods. Heat can reduce the shelf life of products, so don’t expose products to elevated temperatures for extended periods.
Some Cook product packages have a natural-rubber latex warning. Products that have this warning contain latex that can come into direct or indirect contact with patients or operators during normal use. An example of indirect contact is when a fluid touches the latex and then touches the patient. Please contact Customer Support or your local sales representative if you have further questions.
![]() Item Contains or Has a Presence of Natural Rubber Latex
Item Contains or Has a Presence of Natural Rubber Latex
Symbol for an item that contains or has a presence of natural rubber latex. This symbol is used when natural rubber latex is used to make the device or packaging. The symbol is not used for devices containing synthetic rubber. The symbol is intended to warn those who may have allergic reactions to natural rubber latex.
| Language | Definition |
|---|---|
| English | Item contains or has a presence of natural rubber latex. |
| České | Obsahuje přírodní latex nebo jeho stopy |
| Dansk | Indeholder eller kan indeholde spor af naturgummilatex |
| Nederlands | Bevat natuurlijke rubberlatex of natuurlijke rubberlatex aanwezig |
| Français | Contient du latex de caoutchouc naturel ou présence de latex de caoutchouc naturel |
| Deutsch | Enthält Latex aus Naturkautschuk |
| Ελληνικά | Περιέχεται ή υπάρχει λάτεξ από φυσικό καουτσούκ. |
| Magyar | Összetevőként vagy csomagolásában természetes gumilatexet tartalmaz |
| Italiano | Contiene o presenta tracce di lattice di gomma naturale |
| 日本 | 天然ゴムラテックスを含む |
| Norsk | Inneholder eller kan inneholde spor av naturgummilateks |
| Polski | Zawartość lub obecność lateksu z kauczuku naturalnego |
| Português | Contém ou está presente látex de borracha natural |
| Español | Contiene o presenta látex de caucho natural |
| Svensk | Innehåller eller förekomst av naturgummilatex |
| 中文 | 含有或存在天然乳胶 |
| 繁體中文 | 含有或存在天然乳膠成分 |