February 5th, 2024
Bloomington, Ind. — Cook Medical’s Motion Hybrid Wire Guide is now commercially available in Canada. This two-in-one wire guide is an excellent choice for clinicians in urological specialties because it functions as both an access wire guide and a safety…
January 31st, 2024
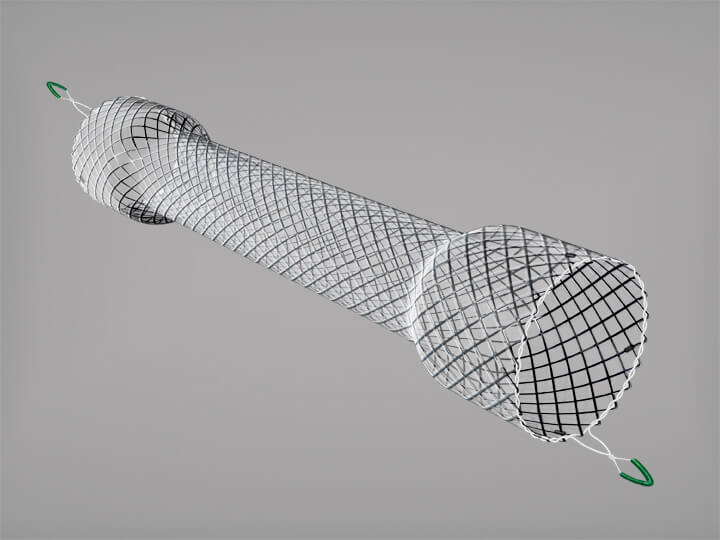
CAUTION - INVESTIGATIONAL DEVICE. Limited by United States law to investigational use. Bloomington, Ind. — Cook Medical today announced the first patient treated in the clinical study of the ZENITH® FENESTRATED+ Endovascular Graft (ZFEN+) in the United States. The procedure was performed…
January 24th, 2024

Bloomington, Ind. — Cook Medical today announced the sale of the remaining Otolaryngology, Head & Neck Surgery (OHNS) product lines, in the latest of a series of strategic decisions aligned to its 5-year vision. Cook signed an agreement with C2Dx,…
January 8th, 2024
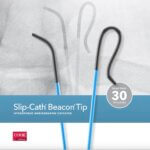
Bloomington, Ind. — Cook Medical’s Slip-Cath® Beacon® Tip Hydrophilic Selective Catheter is now available for use in the U.S. and Canada. Slip-Cath…
December 18th, 2023

Bloomington, Ind. — Cook Medical won the 2023 Loyal Partner Supplier Award from Capstone Health Alliance for outstanding work from Cook’s MedSurg division. Cook is proud…
November 7th, 2023

The last several years have brought significant change to Cook. To ensure that we are positioned to be successful in this new world, our global leadership teams have implemented a new vision and strategic plan that were informed by extensive…
November 1st, 2023
Bloomington, Indiana — In alignment with its 5-year strategic plan, Cook Medical announced today that CooperCompanies has acquired select products from Cook’s Maternal Fetal Medicine portfolio, as well as gynecological surgery products, and Doppler monitor technology. CooperCompanies (Nasdaq: COO), a…
October 18th, 2023
Bloomington, Ind. — The US Department of Veterans Affairs (VA) has awarded Cook Medical a Next Generation Implant Contract for implanted medical devices. This contract will streamline the procurement process for the VA supply chain team. This contract is another…
September 29th, 2023

Bloomington, Ind. — Cook Medical is pleased to promote Barry Slowey to vice president and chief sustainability officer. His new position was effective September 8, 2023. In his new appointment, Barry will provide leadership and implement the goals of Cook’s Social…
September 26th, 2023

Bloomington, Ind. — At the 2023 Vizient Connections Summit, Cook Medical won the Vizient Supply Assurance Supplier of the Year Award. This award demonstrates Cook’s transparency and commitment to communication during the supply chain process with healthcare organizations that are…
September 1st, 2023

Bloomington, Ind. — Cook Medical is pleased to promote Amber Beauchamp to vice president, global human resources at Cook Group and Cook Medical effective September 1, 2023. Beauchamp has more than 17 years of HR experience in multiple industries. She…
August 7th, 2023
Gina W. shares her diagnoses story and advocates for disease awareness In an open, honest, and, at times, emotional conversation, Gina W., a national sales manager for Cook, sat down with interviewer Kelly R., a Clinical Training manager for Cook,…
July 25th, 2023

Bringing the latest in medical technologies to the youngest patients INDIANAPOLIS and WEST LAFAYETTE, Ind. – Purdue University, the Indiana University School of Medicine and medical device company Cook Medical are focusing a new alliance on clinical needs in pediatrics…
July 17th, 2023
Cook applauds the U.S. Food and Drug Administration’s recent update on paclitaxel-coated devices. We believe this is the best decision for physicians and patients. “We are grateful for the FDA’s latest update on paclitaxel. We applaud decisions that are based…
June 29th, 2023
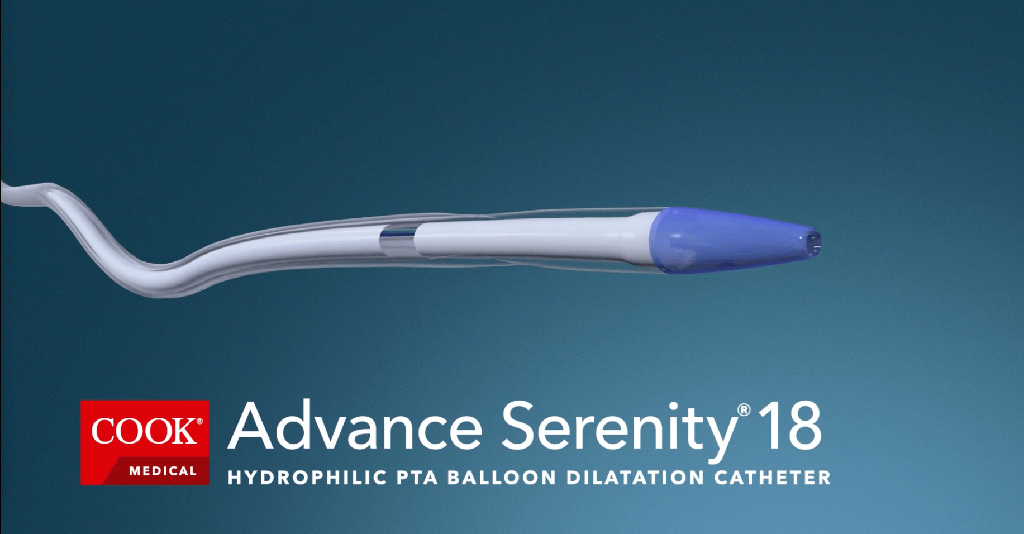
Bloomington, Ind. — As announced at the 2023 Vascular Annual Meeting (VAM), the Advance Serenity® Hydrophilic PTA Balloon Catheter product line is now available with even more options. Interventionalists who perform peripheral intervention procedures in the US and Canada now…
June 26th, 2023

Bloomington, Ind. — Following the publication of an animal study examining the performance of embolization coils in arteries1 in the Journal of Vascular and Interventional Radiology (JVIR) in 2019, a second, similar study,2 published in the May 2023 issue of…
June 23rd, 2023
CAUTION: Investigational device. Limited by United States law to investigational use. Bloomington, Ind. — The U.S. Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an Investigational Device Exemption (IDE) study on the Zenith Fenestrated+ Endovascular…
June 12th, 2023

For five years, the Cook Medical headquarters in Bloomington, Indiana has hosted e-recycling events. We held it again this year, and the tradition has recently sparked other electronics recycling events at other Cook locations. It’s a part of who we…
June 2nd, 2023
Bloomington, Ind. — Cook Medical is proud to achieve a transparency partner badge from the Healthcare Industry Resiliency Collaborative (HIRC). HIRC is a nonprofit healthcare supply chain trade association. Its mission is to support supply chain best practices for better…
May 15th, 2023

The email below, along with a video from our president and an FAQ document, was sent to all Cook Medical employees on Monday, May 15 at 1pm EST. Memo header: Company announcement: A difficult step for our strategic success…
May 4th, 2023

Bloomington, Ind. — For the third year in a row, Cook Medical has received a Greenovation award from Kimberly-Clark’s RightCycle Program. The award is for Cook’s continued sustainability leadership and participation in a landfill diversion program for nitrile gloves. [caption…
May 2nd, 2023

Jennifer Ludden and Marisa Peñaloza shared Cook Medical's housing initiative on NPR's Morning Edition. The piece is called "Would you live next to co-workers for the right price? This company is betting yes." NPR: Listen here On Tuesday, May 2,…
April 28th, 2023

Bloomington, Ind. — Cook Medical announces the first patient treated in a clinical study to evaluate a new venous valve designed for treating chronic venous insufficiency. The patient was treated by Dr. Mauricio Alviar, vascular surgeon and principal investigator, of…
April 3rd, 2023
Bloomington, Ind. — Cook Medical has received a contract with Vizient for endoscopy devices. This new agreement will allow Cook to continue to offer our endoscopy devices at negotiated pricing terms to healthcare facilities that are members of Vizient. With…
March 2nd, 2021

Bloomington, Ind. – A new medical device manufacturing facility isn’t just bringing 100 new jobs to Indianapolis—the site will also boost the economy with a goal of contracting with 100% local minority-owned construction companies. Harmon Construction, a third-generation family-owned contractor,…
March 1st, 2021
Bloomington, Ind. — Cook Medical’s Zenith® Fenestrated+ Endovascular Graft (ZFEN+) product has received Breakthrough Device designation from the US Food and Drug Administration (FDA). This designation is granted for devices that have the potential to provide more effective treatment or…
January 11th, 2021
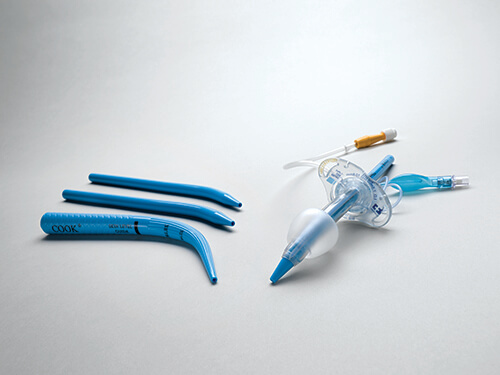
Bloomington, Ind. — Cook Medical today announced that the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays are commercially available to physicians in the United States and Canada. This product is a new iteration of the Ciaglia Blue Rhino…
January 6th, 2021
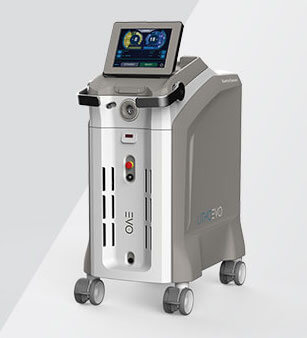
Bloomington, Ind. – The Litho EVO holmium laser is now available through Cook Medical in the United States, Austria, Germany, France, Ireland, Switzerland, and the United Kingdom. The Quanta Litho EVO laser is now available through…
December 28th, 2020
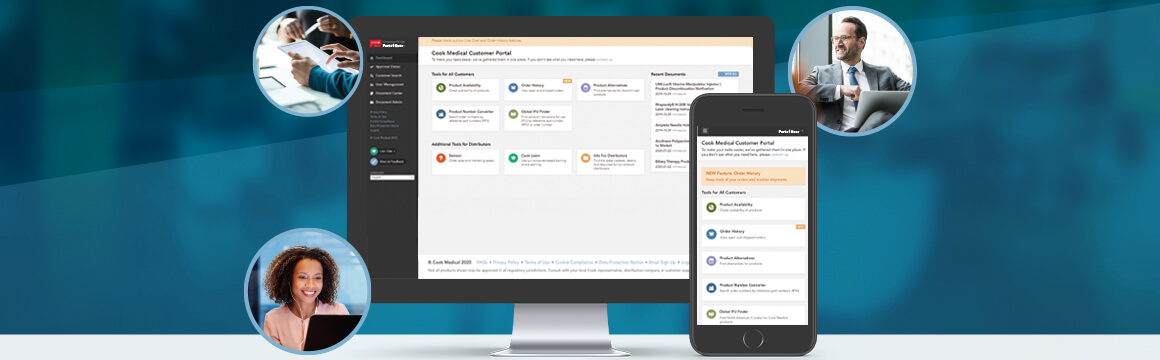
Bloomington, Ind. – Cook Medical is pleased to announce the launch of a new Customer Portal to provide an enhanced digital and self-serve customer experience. “The core focus…
November 20th, 2020
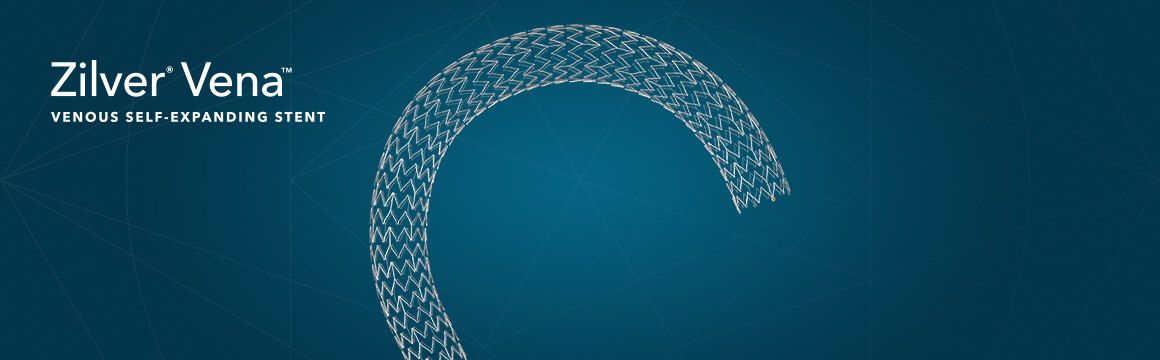
Bloomington, Ind. – Cook Medical today announced that the Zilver® Vena™ Venous Self-Expanding Stent is commercially available to physicians in the United States. Zilver Vena is a self-expanding stent designed to meet the needs of patients suffering from symptomatic iliofemoral…
November 18th, 2020

View news coverage of this announcement below. How a Unique Partnership Between Neighbors, a Medical Device Company, Government, and Non-Profits is Changing an Indianapolis Community Bloomington, Ind. – A unique partnership designed to bring jobs, free education, wrap around services…
November 15th, 2020

Bloomington, Ind. – At this year’s Vascular Interventional Advances (VIVA) conference, Dr. Anthony Comerota presented data on the Zilver® Vena™ Venous Self-Expanding Stent that demonstrates durable patency and symptom relief in patients over an extended period of time. [caption id="attachment_7957"…
October 26th, 2020
Bloomington, Ind. – Cook Medical announced today that the Hercules® 100 Transnasal Esophageal Balloon is now commercially available to physicians in the US. This product is the first balloon designed specifically for transnasal esophageal procedures. With the Hercules 100, ENT…
October 15th, 2020

Bloomington, Ind. – Cook Medical today announced that the Zilver® Vena™ received FDA premarket approval (PMA) in the United States. The product is expected to be commercially available to physicians in the U.S. in Q4 2020. Zilver Vena is a…
October 6th, 2020

Bloomington, Ind. – Cook Medical today announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has granted a new technology add-on payment (NTAP) for Hemospray® Endoscopic Hemostat. Effective on October 1, 2020, the designation will provide eligible hospitals with…
September 12th, 2020
Bloomington, Ind. – At this year’s Cardiovascular and Interventional Radiological Society of Europe (CIRSE) conference, Michael D. Dake, MD, presented a prediction model for treatment outcomes for patients treated with Zilver PTX. The model includes data generated from more than…
September 2nd, 2020
Cook is a family company founded in 1963 and has grown into a global, multicultural organization. We were founded on core values of mutual respect, acting with integrity, and deeply committing to the quality of products and services we provide…
August 13th, 2020

Bloomington, Ind., and Shaumburg, Il. – Dave Reed, retired vice president of Healthcare Business Solutions at Cook Medical, was recently inducted into the Hall of Fame for Healthcare Supply Chain Leadership by the Bellwether League, Inc. This year, the Bellwether…
August 3rd, 2020

Bloomington, Ind. – A study recently published in BMC Urology compared Cook Medical’s Black Silicone Filiform Double Pigtail Ureteral Stent Set to a traditional polyurethane ureteral stent and found that on two separate patient surveys, the black silicone stent was…
June 23rd, 2020
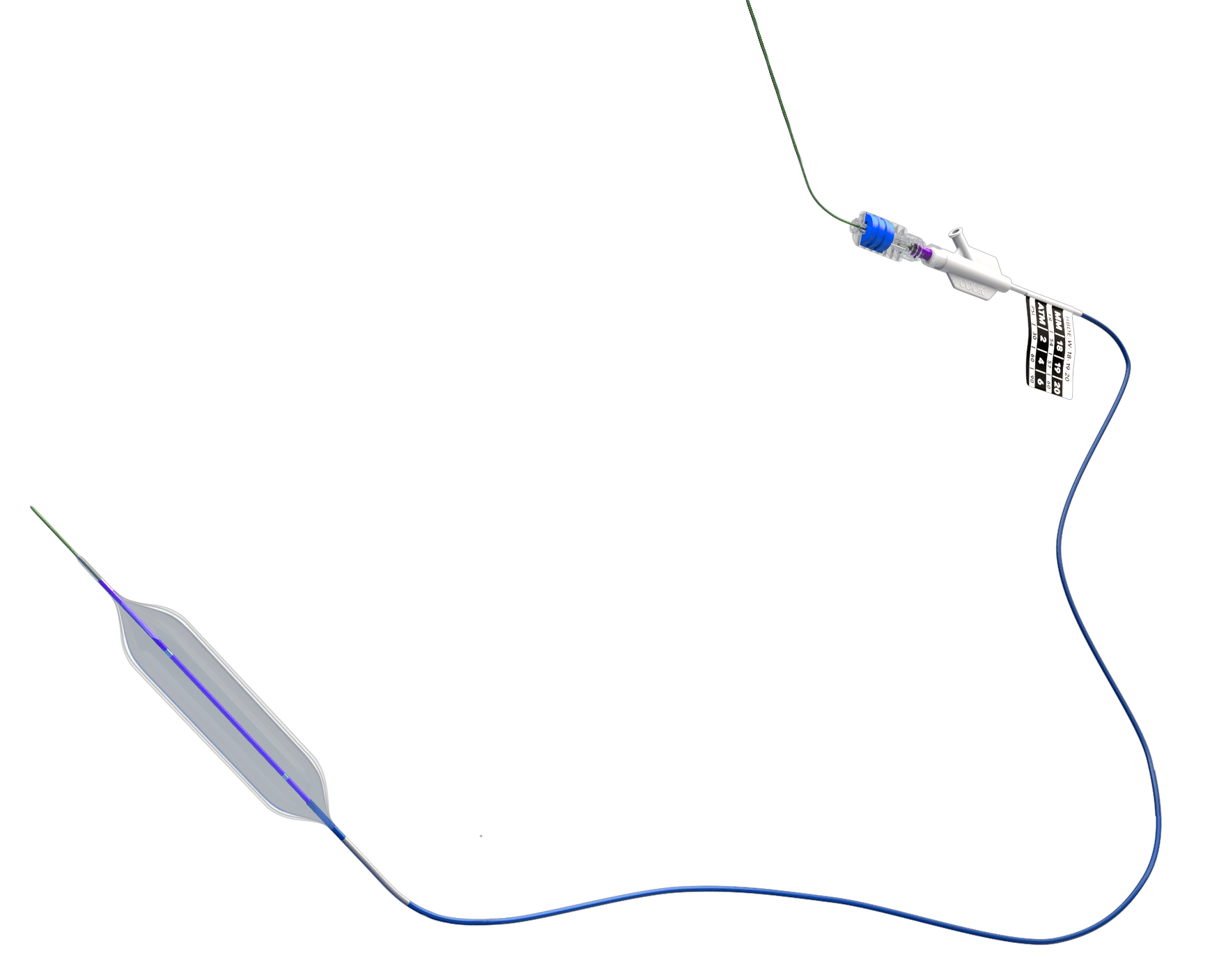
Bloomington, Ind. – Cook Medical today announced that the Advance Serenity™ Hydrophilic PTA Balloon Catheter is now available to physicians in the United States. The catheter is manufactured by Surmodics and distributed by Cook Medical. Advance Serenity is a hydrophilic-coated…
June 22nd, 2020

The Indianapolis Business Journal highlighted Cook Medical and Cook Group as one of the top 50 privately owned companies in Indiana. Cook Group ranked third in the state of Indiana this year. Reporter Sam Stall interviewed several Cook executives about…
June 8th, 2020

Bloomington, Ind. – Cook Medical is proud to announce the release of two products. The Transluminal Biliary Biopsy Forceps Set (BBFS) and a new 40-centimeter delivery system line extension of the existing Zilver 635® Biliary Stent (ZIB) are both now…
June 3rd, 2020

This is a painful time in our country. It’s unfathomable that tragedies such as the deaths of George Floyd, Breonna Taylor, and Ahmaud Arbery continue to happen. Sadly, as people of color know all too well, they do happen every…
April 30th, 2020
Bloomington, Ind. – Cook Medical and Surmodics, Inc. are proud to announce a new agreement in which Cook Medical will distribute two new Surmodics products, hydrophilic percutaneous transluminal angioplasty (PTA) balloon catheters that can be used over 0.014-inch and a…
April 30th, 2020
First, we want to thank everyone on the front lines fighting this pandemic. We acknowledge the sacrifices you are making to protect your own families while meeting your obligations to patients. Across the global healthcare industry, the COVID-19 pandemic has…
January 29th, 2020
Bloomington, Ind. – Cook Medical and Bentley today announced that they are collaborating in clinical trials involving Cook’s fenestrated and branch stent grafts and Bentley’s covered stents. About Cook Medical Since 1963, Cook Medical has worked closely with physicians to develop technologies…
January 21st, 2020
Bloomington, Ind.—Cook Medical is pleased to announce a new contract with the US Department of Veterans Affairs (VA). The contract, which went into effect on October 21, 2019, includes products such as the Zilver® PTX® Drug-Eluting Peripheral Stent, Zenith® aortic…
January 15th, 2020

Cook Medical's TriForce® Peripheral Crossing Set Bloomington, Ind. – Cook Medical’s TriForce® Peripheral Crossing Set is now commercially available. As of January 2020, these products are available to physicians in the United States to support procedures to treat patients with…
January 13th, 2020
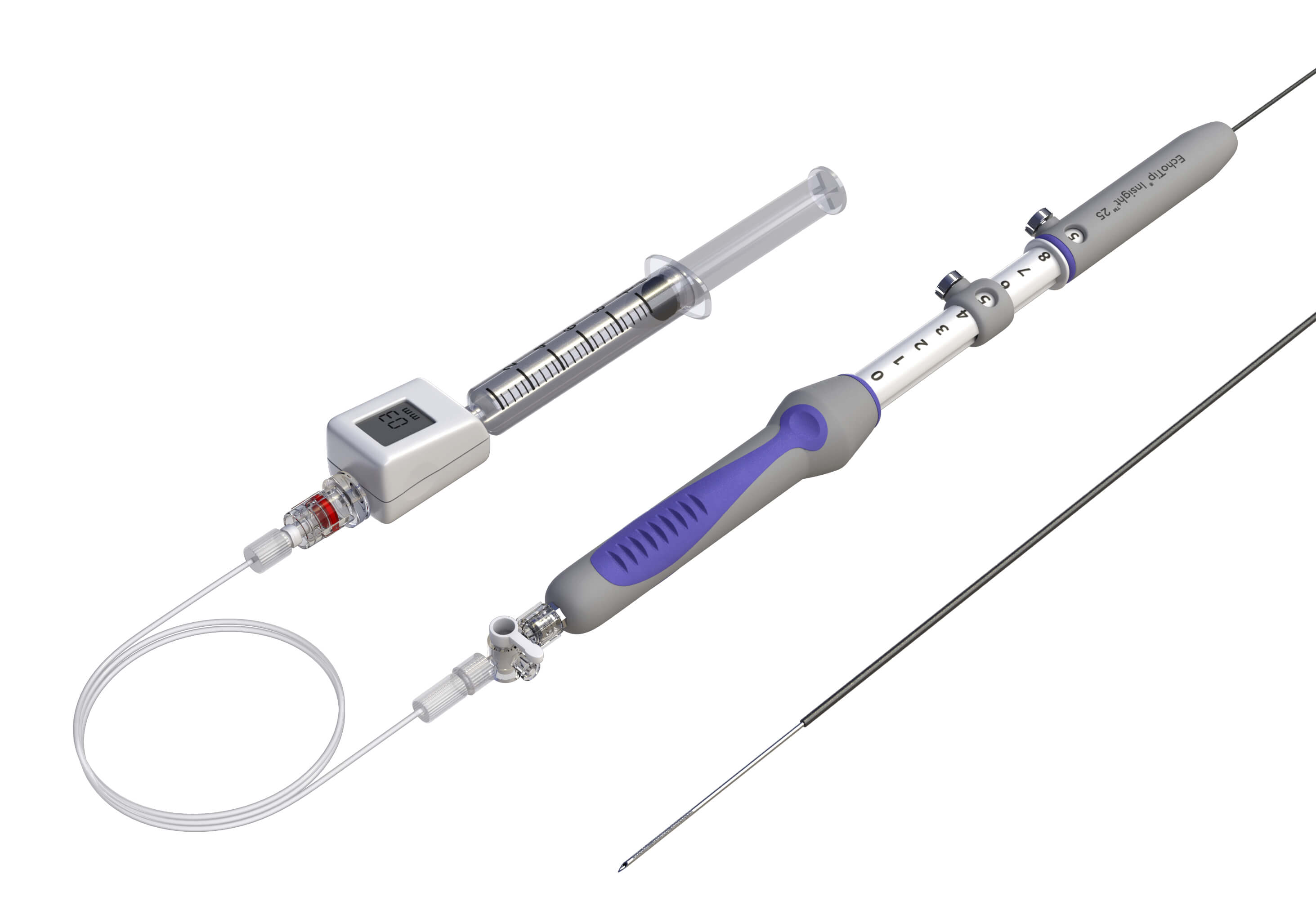
Bloomington, Ind. – The FDA has recently granted Cook Medical a De Novo authorization to market their new device, EchoTip® Insight™ Portosystemic Pressure Gradient Measurement System, in the United States. EchoTip Insight is an endoscopic ultrasound device which allows endoscopists…
January 7th, 2020
Bloomington, Ind. — On January 6, the United States District Court for the Southern District of Indiana vacated in its entirety a February 1, 2019, judgment against Cook Medical in the inferior vena cava (IVC) filter litigation. In vacating the verdict, the…
December 20th, 2019
Bloomington, Ind. — Today, Cook Medical applauds the successful efforts of legislators from the U.S. Senate and House of Representatives to repeal the medical device excise tax. “Repealing the medical device tax will help bring new life-saving devices to patients around the…
December 3rd, 2019

Bloomington, Ind. — Cook Medical’s innovative Hemospray® product received the 2019 Healio Disruptive Innovators Award in the Industry Breakthrough category. Healio, a healthcare news and education organization, presented the awards on October 27 at the American College of Gastroenterology Annual Scientific…
November 19th, 2019

Bloomington, Ind. – At this year’s VEITHsymposium®, Dr. Marc Bosiers presented data that show that patient treatment with the Zilver® PTX® stent has several benefits when compared to traditional bypass surgery. The data, which were gathered from a randomized controlled…
November 12th, 2019

Hong Kong — Cook Medical announced today that Jean-Marc Creissel has been named vice president and director of Asia-Pacific. In his new role, Creissel will lead the Asia-Pacific teams to transform Cook Medical into…
November 5th, 2019

Bloomington, Ind. — At this year’s Vascular Interventional Advances (VIVA) conference, Dr. Michael D. Dake presented data on Zilver® PTX® that supports the device’s benefits across different patient groups. …
October 29th, 2019
Bloomington, Ind. – Cook Medical and Quanta System have entered into an agreement in which Cook will distribute in some territories a comprehensive offering of Quanta’s holmium and thulium laser systems, laser fibers and accessories. This collaboration will ensure that…
October 28th, 2019
Earlier today, a decision was made in Philadelphia against Rex Argon, an IVC filter device manufacturer. Cook Medical’s IVC filters are quality products that have saved thousands of lives and are critical to patient well-being. Our design and materials are different from the…
October 14th, 2019

Phoenix, Ariz. — Cook Medical is proud to announce that Dave Reed, vice president of Healthcare Solutions, received the Chuck Lauer Award at the Integrated Delivery Network (IDN) Summit on September 10. Dave Reed (left) receives…
October 7th, 2019

Bloomington, Ind. — Forbes named Cook Medical one of America’s Best Employers for Women 2019. We are honored to receive this award in addition to the Forbes rankings as one of America’s Best Employers for both 2018 and 2019. To rank…
October 2nd, 2019

Abu Dhabi, UAE — Shayna Martin, global brand marketing director for Cook Medical’s Urology specialty, will receive the Endourological Society 2019 Industry Award during the society’s…
September 10th, 2019

Barcelona, Spain — Michael D. Dake, MD, presented at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) conference regarding new data on the use of Zilver® PTX®, Cook Medical’s paclitaxel-coated stent for peripheral arterial disease (PAD). Dr. Dake is the…
August 28th, 2019

Bloomington, Ind. — Cook is pleased to announce that Rose Kelly-Falls has joined Cook Medical as global leader of procurement and supply chain. Kelly-Falls and…
August 20th, 2019
Bloomington, Ind. — Cook Medical recently released the second generation of the 2.6 Fr CXI Support Catheter with platinum-iridium marker bands. The CXI catheter is used in small-vessel anatomy or superselective anatomy for diagnostic and interventional procedures, including peripheral use. “We…
August 7th, 2019
Today, FDA updated its communication to healthcare providers on paclitaxel coated devices. Based on current information, FDA recommends that physicians discuss the risks and benefits of all available peripheral arterial disease (PAD) treatment options with patients and determine if the…
June 26th, 2019
Last week, during FDA’s public meeting of the Circulatory System Devices Panel, there was an important discussion on patient safety and mortality rates associated with paclitaxel coated medical devices. During the meeting, Cook Medical presented 5-year clinical data on our…
June 24th, 2019
Bloomington, Ind. — A newly published prospective, randomized controlled trial concluded that 20 gage fine needle biopsy (FNB) needles consistently outperformed 25 gage fine needle aspiration (FNA) needles in terms of histological yield and diagnostic accuracy, in pancreatic and non-pancreatic…
June 4th, 2019
Bloomington, Ind. — Today, Cook Medical was honored as one of Bloomington’s Best Places to Work by Bloomington Economic Development Corporation (BEDC), The Mill and The Herald-Times. This is the second consecutive year that Cook has received recognition for its…
May 20th, 2019
Bloomington, Ind. — A new, multicenter study on Cook Medical’s Hemospray Endoscopic Hemostat device was published in the April 2019 edition of Gastrointestinal Endoscopy. The prospective, nonrandomized, Cook sponsored study was conducted at 4 tertiary care centers in Canada and involved 50…
May 18th, 2019
Bloomington, Ind. – Cook Medical and Ambu A/S have agreed on terms to enter into a partnership under which Cook will distribute Ambu’s single-use, disposable duodenoscope in the U.S., following FDA clearance. The scope is currently being developed by Ambu…
April 18th, 2019
Bloomington, Ind. — This week, Cook Medical made patient-level data from the Zilver® PTX® randomized control trial (RCT) available by request on cookmedical.com. Cook provides this data to encourage further collaboration with researchers to benefit patients with peripheral arterial disease. “We…
April 17th, 2019
Bloomington, Ind. – Today, Forbes announced that Cook Medical has ranked 48 of 500 large companies in the America’s Best Employers of 2019 listing. This is the second year that Cook Medical has been recognized with this honor. “It is…
March 28th, 2019
Bloomington, Ind. — Following the recent FDA approval of Cook Medical’s Zenith Dissection Endovascular System, Cooper University Health Care in Camden, New Jersey, has treated the first patient in the US with the device as part of Cook’s US commercial launch.…
March 25th, 2019

Bloomington, Ind. – A new animal study concludes that fewer fibered embolization coils are needed to achieve acute occlusion when compared with similar bare metal coils. The study was presented today at an industry sponsored symposium during the 2019 annual…
March 22nd, 2019
Dear colleagues, Over the past few months, there has been a lot of discussion regarding the use of paclitaxel for the treatment of peripheral arterial disease. We want to share with you our perspective and some important information on this…
February 6th, 2019
Jury rejects request for tens of millions of dollars and punitive damages Bloomington, Ind. – Despite overwhelming evidence to support the safety and benefits of the Celect™ Vena Cava Filter, a jury in Indianapolis, Indiana, ruled in favor of the…
February 4th, 2019
Bloomington, Ind. — Today, Cook Medical announced its recent approval from the U.S. FDA for its Zenith Dissection Endovascular System. The system, consisting of a proximal stent-graft component and a distal bare stent component, provides physicians a less invasive alternative to…
January 22nd, 2019
Findings contradict meta-analysis linking paclitaxel-coated devices and increased mortality Bloomington, Ind. – Today at the Leipzig Interventional Course (LINC), Cook Medical participated in significant discussion about the use of paclitaxel to treat patients suffering from PAD. Through several presentations to…
January 2nd, 2019
Winston-Salem, N.C. – Today, Cook Medical announced that it has completed an agreement with Whitaker Park Development Authority Inc. to acquire a long-standing facility at Winston-Salem’s Whitaker Park. The agreement was completed on December 27, 2018. Cook will begin converting a…
November 6th, 2018
Study finds 91 percent freedom from TLR at one year and 76 percent at five years, including complex lesions Bloomington, Ind. – Cook Medical introduced the results from an aggregated data analysis at the Vascular Interventional Advances (VIVA) conference, highlighting…
October 29th, 2018

Bloomington, Ind. — Last week, Cook Medical was recognized as one of the best places to work in Bloomington. The honor comes from the Bloomington Economic Development Corporation in partnership with…
September 24th, 2018
Bloomington, Ind. – Cook Medical announced that a new 5 mm diameter version of Zilver® PTX® was approved by the FDA. It is the first 5 mm drug eluting stent in the U.S. with lengths available up to 140 mm…
September 10th, 2018
Bloomington, Ind. – Cook Medical today announced that it received a close-out letter from the U.S. Food and Drug Administration resolving a 2014 warning letter for processes related to the quality system at the company’s manufacturing facility in Bloomington, Indiana.…
August 8th, 2018
Bloomington, Ind. –Cook Medical has introduced the 140 mm-length Zilver® PTX® Drug-Eluting Peripheral Stent in both 6 and 7 mm diameters in the U.S. The longer length comes after an expanded indication approval by FDA to treat total lesion lengths up…
July 24th, 2018
Bloomington, Ind. – Cook is pleased with bipartisan efforts by the U.S. House of Representatives to help bring new life-saving medical devices to patients by passing H.R. 184, the Protect Medical Innovation Act. This legislation would permanently repeal the medical…
June 12th, 2018
Winston-Salem, N.C. – Today, Cook Medical announced a letter of intent was signed with the Whitaker Park Development Authority Inc. to acquire a long-standing facility at Winston-Salem’s Whitaker Park. A portion of the R.J. Reynolds building will transform into a…
May 24th, 2018
Bloomington, Ind. – Despite overwhelming evidence to support the safety and benefits of the Celect™ Vena Cava Filter, today a Houston, Texas jury ruled in…
May 17th, 2018

Bloomington, Ind. – Last week, Dave Reed was recognized as a leader in healthcare operations by the Global Healthcare Exchange (GHX). The Supply Chain Leadership award recognizes companies and individuals who make a difference in healthcare, transforming supply chain automation,…
May 7th, 2018

Bloomington, Ind. – The FDA has granted Cook Medical approval to market Hemospray®, in the United States. An endoscopic hemostat, Hemospray achieves hemostasis with a proprietary inorganic powder. Bleeding in the gastrointestinal tract is a…
May 4th, 2018

Bloomington, Ind. – Cook Medical has announced Ross Harvey as the new director of global Customer Support & Delivery (CSD). In this role, Harvey will oversee the global customer service teams, global distribution centers, and warehouses. These teams will work…
May 2nd, 2018

Bloomington, Ind. — Yesterday, Forbes announced Cook Medical as one of America’s Best Employers of 2018. Cook Medical ranked 44 on the list of 500 midsize companies and ranked third of 25 in the midsize healthcare equipment and suppliers' category. This is…
April 20th, 2018

Bloomington, Ind. – Today, Christina Anné was named vice president of Distribution Channel Management (DCM) for Cook Medical. The DCM team will be dedicated to serving the needs of Cook’s distributors around the world and to ensure compliant and ethical…
April 2nd, 2018
Bloomington, Ind. – Today, Cook Medical announced that physicians in the U.S. and Canada are once again able to order the Beacon® Tip Torcon NB® Advantage 5 Fr Catheter, used in angiographic procedures. In 2015 and 2016, Cook recalled all…
March 19th, 2018
Bloomington, Ind. – Cook Medical’s Cellvizio® Confocal Laser Endomicroscopy (CLE) System and the Confocal Miniprobes™ have received additional 510(k) clearance from the U.S. Food and Drug Administration (FDA). Additional data provided in multiple peer-reviewed medical journals paved the way for…
March 15th, 2018

Bloomington, Ind. – District Court Judge Richard L. Young entered judgment in favor of Cook Medical in the second bellwether case on IVC filters.…
March 7th, 2018

Bloomington, Ind. – Today, Cook Medical announced Dan Kaiser as the leader of global research and development (R&D). This dedicated engineering team will connect product development more closely to customer needs and accelerate the process of getting new products to…
February 28th, 2018
Bloomington, Ind. – Today, Cook Medical announced the leaders of its two new business divisions. Mark Breedlove has been named vice president of Cook’s Vascular division and DJ Sirota has been named vice president of Cook’s MedSurg division. The new…
February 20th, 2018

Bloomington, Ind. – Today, Cook Medical announced key changes that simplify its organizational structure to better support customers. These changes realign the current sales, marketing, research and development, and customer service teams in addition to establishing new distribution channel management…
January 22nd, 2018
Bloomington, Ind. — Today, Cook Medical applauds the successful efforts of legislators in both the U.S. Senate and House of Representatives to suspend the medical device excise tax for two years. This action is a continuation of the previous two-year suspension of…
November 9th, 2017
Bloomington, Ind. – Today a jury returned a verdict in the first bellwether trial on IVC filters. An Evansville, Indiana, jury returned a unanimous verdict in favor of Cook after a three week trial. “We are pleased with this outcome.…
September 22nd, 2017

Bloomington, Ind. – Jean-Marc Creissel received the Endourology Society 2017 Industry Award during the society’s annual World Congress of Endourology and SWL in Vancouver. Creissel is vice president of Cook Medical’s…
September 5th, 2017
Bloomington, Ind. – Cook Medical’s Urology business announced that it will begin distributing the Cellvizio® Confocal Laser Endomicroscopy (CLE) System manufactured by Mauna Kea Technologies. With the Cellvizio system, a physician can visualize the internal microstructure of tissues in real…
August 24th, 2017
On June 22, 2017, Cook Medical initiated a correction to the Instructions For Use (IFU) for the Zenith Alpha™ Thoracic Endovascular Graft. This correction removed the indication for blunt thoracic aortic injury, also known as BTAI or “transection” of the…
August 14th, 2017
Bloomington, Ind. – Today, Cook Group announced a two-acre land donation to the Washington Township fire…
August 2nd, 2017
Bloomington, Ind. – Today, Cook Group announced a new partnership with Western Governors University (WGU), anonprofit online university. All U.S. Cook employees will be eligible for a five percent tuition discount for bachelor’s and master’s degree programs in IT, business,…
July 10th, 2017
Bloomington, Ind. – June marked the first anniversary of the My Cook Pathway program, Cook Group’s workforce initiative. The program provides Cook employees with education and career development opportunities through financial assistance. Cook first launched the program by announcing partnerships with…
June 21st, 2017
The Herald-Times, a local Bloomington, Indiana paper, profiled Cook’s High School Equivalency program in a feature. Reporter Kurt Christian sat down with Cook leadership and recent program graduate Nycole to capture stories and successes of the adult education initiative. Read…
June 13th, 2017

Bloomington, Ind. – April marked the first anniversary of Cook’s tutoring program in partnership with Bloomington’s Crestmont Boys & Girls Club. Over the past year, 30…
May 25th, 2017

West Lafayette, Ind. – Jennifer Kerr, president of Cook Research, was named to the 2017 class of Hoosier Fellows through the Randall L. Tobias Center for Leadership Excellence at Indiana University. Participants in the Hoosier Fellows program come from across…
May 19th, 2017

Bloomington, Ind. – Students from Ivy Tech Community College Bloomington have collaborated with Cook Medical and Zumasys through real-life computer programming coursework on jBASE, a database management system used by manufacturers worldwide. Zumasys, a cloud computing platform and owner of…
May 10th, 2017
Bloomington, Ind. – Cook Medical is pleased to announce the sale of its comprehensive family of vertebroplasty products to IZI Medical Products, LLC. This sale includes the Duro-Ject® Osteo-Site®, Osteo-Force® and Vertefix® brands in addition to other needles, injectors and…
May 8th, 2017
Bloomington, Ind. – Cook Medical’s Endoscopy business unit redefines access with the introduction of their latest wire guide, the next generation Acrobat® 2 Calibrated Tip Wire Guide. The Acrobat 2 provides the tip flexibility needed for ductal navigation. "We’re very…
May 2nd, 2017
Bloomington, Ind. – Last week, Cook Medical was named Industry Partner of the Year by the Indiana Association for Adult Education for its High School Equivalency (HSE) program. In 2016, Cook partnered with Broadview Learning Center for Adults through Monroe…
April 7th, 2017
Bloomington, Ind. – Pete Yonkman, president of Cook Group and Cook Medical, announced a fund to help small tech companies attract new employees at this weekend’s Combine event…
March 29th, 2017
Winston-Salem, NC. — Twin City Track Club announced today that Cook Medical will become the Presenting Sponsor for the Beat the Heat 5K events, which will be held on July 22, 2017 starting at 6:30 p.m. “We are honored that…
March 27th, 2017
Bloomington, Ind. – Today Governor Eric Holcomb honored Cook Medical as a 2017 recipient of the Governor’s Half Century Business Award at the Indiana Statehouse in Indianapolis. This award is in recognition of the fact that for more than 50…
March 13th, 2017
West Lafayette, Ind. – Cook Medical is pleased to announce the promotion of Umesh Patel to president of Cook Biotech Incorporated. As president, Patel will oversee the various functions at Cook Biotech located in West Lafayette, Ind. Patel’s understanding of…
March 7th, 2017
Bloomington, Ind. – Ivy Tech Community College Bloomington was awarded a Perkins Competitive Grant from the Indiana Department of Education in the fall of 2016 to increase rural career and technical education pathways in biotechnology. The $85,000 grant is being…
February 23rd, 2017

Bloomington, Ind.— Cook Medical has rounded out the Universa line with the introduction of two new sets for percutaneous urinary drainage. Each set includes a catheter and accessories for specific procedures. These…
January 4th, 2017
Bloomington, Ind.— Cook Medical announced the introduction of a two-in-one wire guide, the Motion™ Hybrid Wire Guide. This wire guide combines the features of a nitinol access wire guide and a Teflon™…
November 15th, 2016
BLOOMINGTON, Ind.-- Cook Medical has completed enrollment in the first clinical study of an iliofemoral venous stent conducted in the United States under an FDA-approved Investigational Device Exemption (IDE). The VIVO Clinical Study is a prospective, non-randomized, multi-center study intended…
November 11th, 2016
Winston-Salem, N.C. – On November 17, Forsyth Tech Community College’s 2016 SciTech Lecture Series will focus on the development process of medical devices. Barry Slowey, president of Cook Endoscopy/Cook Winston-Salem, and Vihar Surti, director of research and development for Cook Medical’s…
October 20th, 2016
Indianapolis, Ind. – The Indiana Biosciences Research Institute (IBRI) today announced a $1 million research grant from Cook Medical to support the next phase of growth for the Institute dedicated to discovery science and applied research leading to innovation targeting cardio-metabolic…
September 22nd, 2016
Bloomington, Ind. – Today, the Bloomington Chamber of Commerce announced Cook Group as the 2016 Workforce Development Award recipient in recognition the My Cook Pathway program the company launched in June. In partnership with Monroe County Community Schools Adult Education…
August 31st, 2016

Bloomington, Ind. – Cook Medical is pleased to announce that Jean-Marc Creissel has been named vice president of operations for the greater China region. In his new role, Creissel will work with the global and regional teams in APAC to…
August 12th, 2016
FOR IMMEDIATE RELEASE: August 12, 2016 Cook Incorporated 750 Daniels Way, P.O. Box 489 Bloomington, Indiana 47402 1-812-339-2235 www.cookmedical.com DSM Biomedical B.V., the Netherlands, Cook Medical’s supplier of hydrophilic coating for the Roadrunner® Uniglide® Hydrophilic Wire Guide, recalled certain lots…
August 10th, 2016

Cook Group and Cook Medical sat down with The Herald-Times, the local newspaper in Bloomington, Indiana, to discuss Cook's transformation and preparation for healthcare of the future. These interviews resulted in a four-day series by veteran reporter Bill Strother as described…
July 11th, 2016

Bloomington, Ind. – Pete Yonkman, Cook Medical and Cook Group president, appeared on Inside INdiana Business with Gerry Dick on July 10, 2016, to discuss Cook's new partnership with Broadview Learning Center and Ivy Tech Community College to create a…
June 17th, 2016

Bloomington, Ind. – Today, Cook Group, Monroe County Community Schools Adult Education at Broadview Learning Center and Ivy Tech Community College Bloomington announced their partnership to create a new pathway between education and jobs in our community. According to the…
May 2nd, 2016
Cook Medical has initiated a global, voluntary recall of all catheters with Beacon® Tip technology. This recall includes all lots of catheters with the Beacon Tip technology. The catheters were recalled on April 15, 2016 due to complaints of tip splitting…
March 3rd, 2016

Limerick, Ireland – In 2016 Cook Medical will celebrate 20 years of operations in the National Technology Park where it has grown from a primary team of less than a dozen people to a staff of over 800, who are…
March 1st, 2016

Bloomington, Ind. – Cook Group is pleased to announce the promotion of Barry Slowey to president of Cook Endoscopy/Cook Winston-Salem. As president, Slowey will oversee the various functions in Winston-Salem, North Carolina, and will maintain his leadership responsibilities for the…
February 10th, 2016
FOR IMMEDIATE RELEASE: February 10, 2016 Cook Incorporated 750 Daniels Way, P.O. Box 489 Bloomington, Indiana 47402 812.339.2235 www.cookmedical.com On January 6, 2016, Cook Medical initiated a voluntary recall of 360 specific lots of Single Lumen Central Venous Catheters and Pressure Monitoring Sets and…
February 1st, 2016
West Lafayette, Ind. – MED Institute (MED) has been renamed as Cook Research Incorporated (CRI) and remains located in the Purdue Research Park at 1 Geddes Way in West Lafayette, Indiana. CRI supports Cook Medical in the identification, development, testing,…
January 26th, 2016
Bloomington, Ind. ─ In November, following the Food and Drug Administration approval of Cook Medical’s Zenith Alpha™ Thoracic Endovascular Graft, the first patient was treated with the device in the U.S. “The first commercial use of Zenith Alpha Thoracic in…
December 23rd, 2015
Bloomington, Ind. — Cook Medical applauds the successful efforts of legislators in both the U.S. Senate and House of Representatives last week to suspend the burdensome medical device excise tax for two years. This action to suspend the medical device tax…
December 10th, 2015
Bloomington, Ind. – Just weeks after 2015 country approval of Cook Medical’s Zenith Alpha™ Abdominal Endovascular Graft, the University of Western London Health Sciences Centre in London, Ontario treated the first patient with the device as part of Cook’s Canada…
December 2nd, 2015
Bloomington, Ind. – A recent study by Breda et al shows that the Flexor® Parallel™ Rapid Release™ Ureteral Access Sheath (UAS) provides physicians an option to use one wire guide for treatment and diagnostic purposes during flexible ureteroscopy (fURS) procedures.…
November 17th, 2015

Bloomington, Ind. – Cook Medical’s Endoscopy clinical division announces the introduction of the latest addition to the EchoTip ProCore® product line, the EchoTip ProCore 20 gage needle with ReCoil Stylet™. This needle’s flexible design allows physicians to obtain histological samples…
October 12th, 2015
Our hearts are heavy as we mourn the passing of our friend and coworker. On Friday night, Bill Gibbons, our vice president of engineering, and his daughter were involved in a plane crash in eastern Tennessee. Bill was 45 and…
October 9th, 2015
FOR IMMEDIATE RELEASE: October 9, 2015 Cook Incorporated 750 Daniels Way, P.O. Box 489 Bloomington, Indiana 47402 812.339.2235 www.cookmedical.com Purpose of the Press Release: On October 7, 2015, Cook Medical initiated a voluntary recall for select sizes of Beacon® Tip…
September 28th, 2015
Bloomington, Ind. — Dr. Kimihiko Kichikawa, Department of Radiology at Nara Medical University in Japan, reported two-year results of the Zilver® PTX® post-market surveillance (PMS) study on September 27, 2015, in Lisbon, Portugal. Dr. Kichikawa presented initial target data on…
September 28th, 2015
Bloomington, Ind. – Cook Medical’s Otolaryngology-Head and Neck Surgery (OHNS) clinical division announces the introduction of the Advance Salivary Duct Balloon Catheter Set. This product is designed to dilate strictures in the submandibular and parotid salivary ducts. In the company’s…
September 24th, 2015
Bloomington, Ind. — Today, Cook Animal Health, a Cook Medical affiliate, announced its distribution agreement with MILA International, Inc. This agreement, effective immediately, will enable MILA to offer over 100 Cook Medical devices for sale to veterinary clinics and veterinary schools…
September 17th, 2015
Bloomington, Ind. ─ Cook Medical has received premarket approval from the U.S. Food and Drug Administration (FDA) for its lower-profile Zenith Alpha™ Thoracic Endovascular Graft. Zenith Alpha Thoracic is indicated for the endovascular treatment of patients with isolated lesions of…
August 10th, 2015
Bloomington, Ind. – Cook Medical has been recognized as a Gold-Level Fit-Friendly Worksite by the American Heart Association for helping employees eat better…
August 3rd, 2015
FOR IMMEDIATE RELEASE: August 3, 2015 Cook Incorporated 750 Daniels Way, P.O. 489 Bloomington, Indiana 47402 812.339.2235 www.cookmedical.com Purpose of the Press Release: On July 2, 2015, Cook Medical initiated a lot-specific voluntary recall of 2,239 lots of Beacon Tip…
June 18th, 2015
Cook is pleased that the House of Representatives, working in a bipartisan effort, has acted to help patients needing the latest medical technologies, support hospitals struggling to control healthcare expenses, and boost U.S.-based device manufacturers in a global competitive market,…
June 16th, 2015

Bloomington, Ind. — Cook Group announced today that Kem Hawkins, president of Cook Group Incorporated, will retire on July 1 after 34 years with the Cook organization. Hawkins, who has been transitioning his role in the company over the past…
May 22nd, 2015
Bloomington, Ind. — Cook Medical is pleased to be one of the global medical device companies to collaborate internationally with regulatory authorities on the Medical Device Single Audit Program (MDSAP). The value of developing a global program to audit and monitor…
May 4th, 2015
Bloomington, Ind. – Cook Medical announces the introduction of the Hercules® 100 3-Stage Wire-Guided Balloon for esophageal dilation. This product is designed to be used for oral or transnasal esophagoscopy (TNE) helping otolaryngologists effectively treat patients who suffer from dysphagia…
February 26th, 2015
Canton, Ill. – On March 2, the iconic big steam whistle affectionately known as “Big Toot” will return to its original home at the old International Harvester site that is now occupied by Cook Medical and the Cook Polymer Technology…
February 24th, 2015
Bloomington, Ind. – Physicians who treat obstructive salivary gland disorders now have the option of a specialized catheter designed to irrigate the salivary duct and flush out stone fragments. Cook Medical’s SialoCath™ Salivary Duct Catheter is a flexible, soft-tipped, single-lumen…
February 10th, 2015
Bloomington, Ind. — In January 2015, Premier, Inc. members gained access to updated contract product pricing on specialty urology products from Cook Medical. Premier and Cook’s Urology division signed a new contract that is valid from January 1, 2015, to…
January 29th, 2015
Bloomington, Ind. – Tuesday afternoon, the U.S. District Court for the Southern District of Indiana ruled in favor of Cook Medical Incorporated and dismissed a patent infringement lawsuit filed by Endotach LLC, a subsidiary of the patent assertion entity Acacia…
January 26th, 2015

Bloomington, Ind., January 26, 2015 – Cynthia Kretz, who has served as general counsel since 2008, has been promoted to vice president general counsel, the company said today. As vice president, Kretz will continue her responsibility leading the global operations…
November 24th, 2014
Bloomington, Ind. – Urologists now have new options for stone extraction devices when performing percutaneous nephrolithotomy (PCNL), as Cook Medical is launching the Perc NGage® and Perc NCompass® nitinol stone extractors today. The new products complement the Perc NCircle®, which…
November 4th, 2014
Las Vegas, Nev., November 4, 2014 — Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) confirmed long-term patency for patients treated with Zilver PTX. The results were presented today by…
September 24th, 2014

Bloomington, Ind., September 24, 2014 – Connie Jackson, who has served as global vice president of human resources since 2005, has been promoted to senior vice president of human resources, the company said today. As senior vice president, Jackson will continue…
September 24th, 2014

Bloomington, Ind., September 24, 2014 – Cook Medical is pleased to announce that Jean-Marc Creissel has been named vice president of the Urology clinical division. He has served as global leader of the business unit since 2010. As vice president, Creissel will…
August 19th, 2014
For Immediate Release: August 26, 2014 Cook Medical 750 Daniels Way Bloomington, IN 47404 www.cookmedical.com Telephone Number: 812-339-2235 On July 10, 2014, Cook Medical initiated a recall of 696 of its CloverSnare™ 4-Loop Vascular Retrieval Snare devices. The device was…
July 10th, 2014
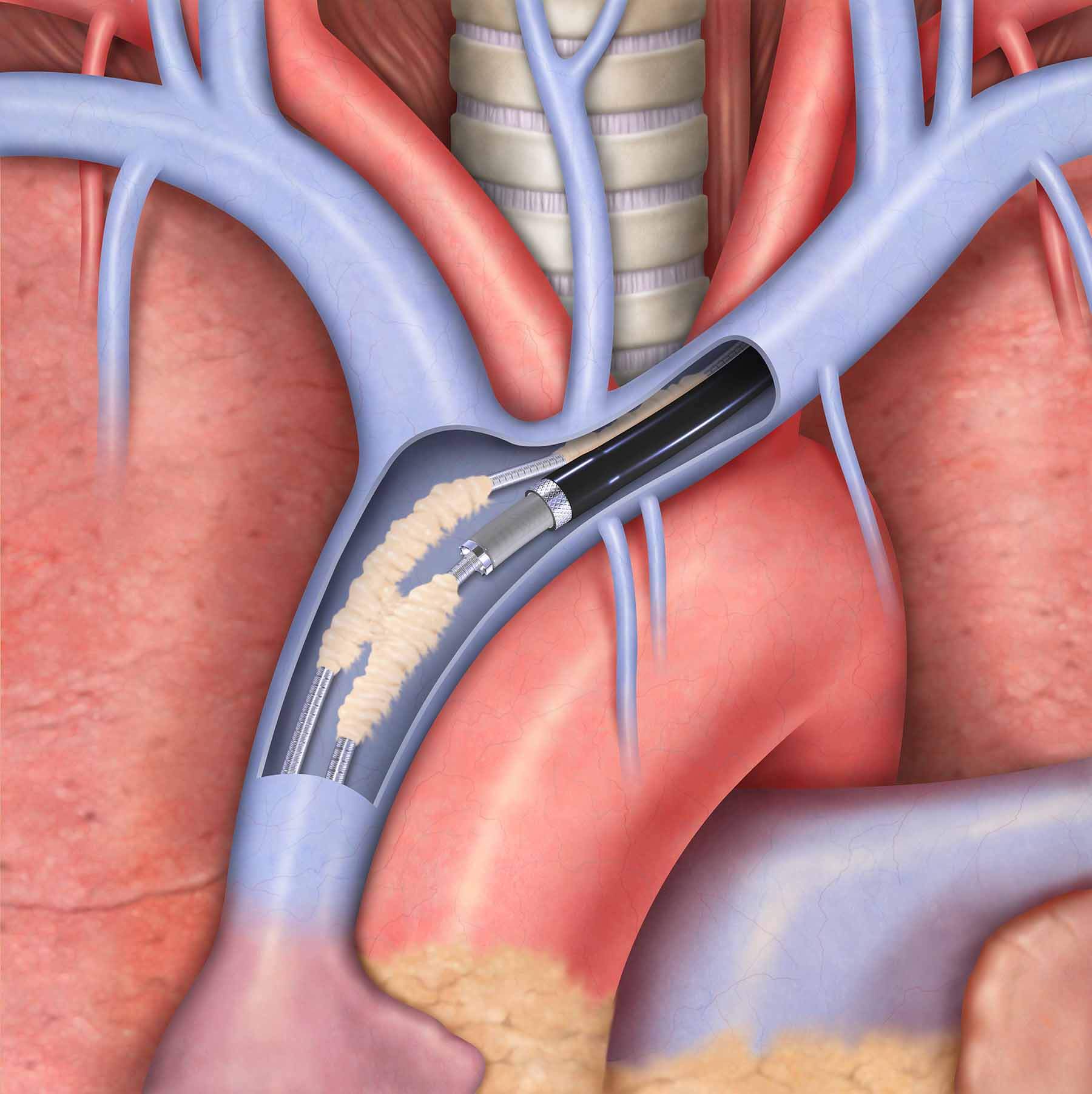
Vandergrift, Pa. — Effective immediately, Cook Medical customers in the United States will again have access to its Evolution® RL and Evolution® Shortie RL Controlled-Rotation Dilator Sheath Sets. Distribution of these products within the U.S. market was suspended for two months while…
June 2nd, 2014
Bloomington, Ind., — Cook Medical is engaged in two clinical studies that will provide additional data on the safety and effectiveness of inferior vena cava (IVC) filters. The first study, the Cook Inferior Vena Cava Filter (CIVC) study, will add to Cook’s…
May 15th, 2014
Orlando, Fla. – Cook Medical will exhibit augmented reality experiences, which is a technology used to simulate use of products, and host a skills challenge at their booth, #1617, at the annual American Urological Association (AUA) meeting. The booth will highlight…
May 6th, 2014
San Francisco, Calif. — A quarter century ago, doctors treating patients with implanted cardiac pacemakers had a big problem. Their patients were outliving the complex electrical devices that gave them an acceptable quality of life. Pacemaker lead wires that deliver electricity…
May 2nd, 2014
Chicago, Ill. – This week is Digestive Disease Week (DDW), and at the world’s largest gathering of physicians and researchers in the gastroenterology field, nine abstracts and other clinical data will be presented on Cook Medical products. Cook’s line of EchoTip®…
April 23rd, 2014

Bloomington, Ind., April 21, 2014 – The Instinct® Endoscopic Clip is now available to gastroenterologists in major global markets. The clip is used to stop gastrointestinal (GI) tract bleeding, which is a condition that can be challenging to treat because of…
April 8th, 2014
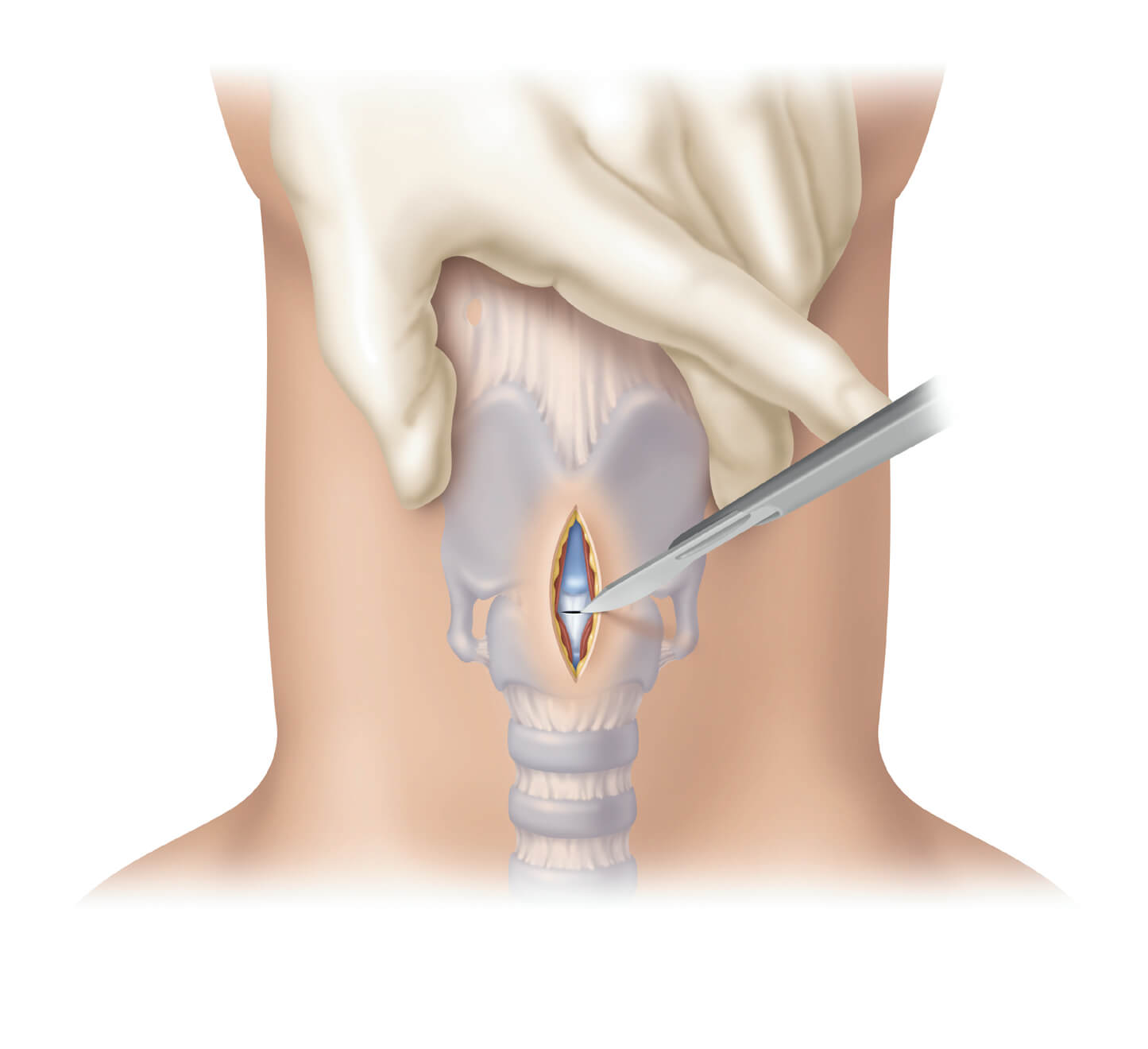
Bloomington, Ind. – In response to the NAP4 report addressing the clinical need for both Seldinger* and surgical cricothyrotomy procedures to be taught side-by-side, Cook Medical today announces a compact surgical set. This cricothyrotomy range expansion allows clinicians to stay up-to-date…
March 3rd, 2014
Bloomington, Ind.— The first ten patients have been enrolled in Cook Medical’s VIVO clinical research study. The study is designed to evaluate the safety and effectiveness of treating symptomatic iliofemoral venous outflow obstruction with the Zilver® Vena™ Venous Self-Expanding Stent. The…
February 20th, 2014

Bloomington, Ind. – CFC Properties, a Cook Group company, welcomes a new vice president of commercial real estate. Ron Walker, former president of the Bloomington Economic Development Corporation (BEDC), is looking forward to his new position. "I am truly…
February 19th, 2014
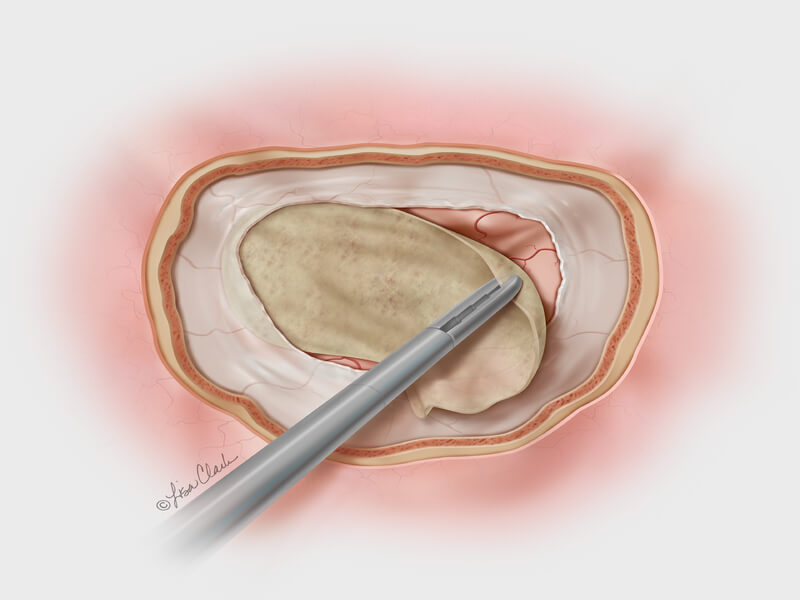
Bloomington, Ind. – Cook Medical has launched a new treatment option for otolaryngologists (ear, nose and throat specialists) who repair the dura mater following cerebrospinal fluid (CSF) leaks at the base of the skull. Cook’s Biodesign® Duraplasty Graft is the…
February 12th, 2014
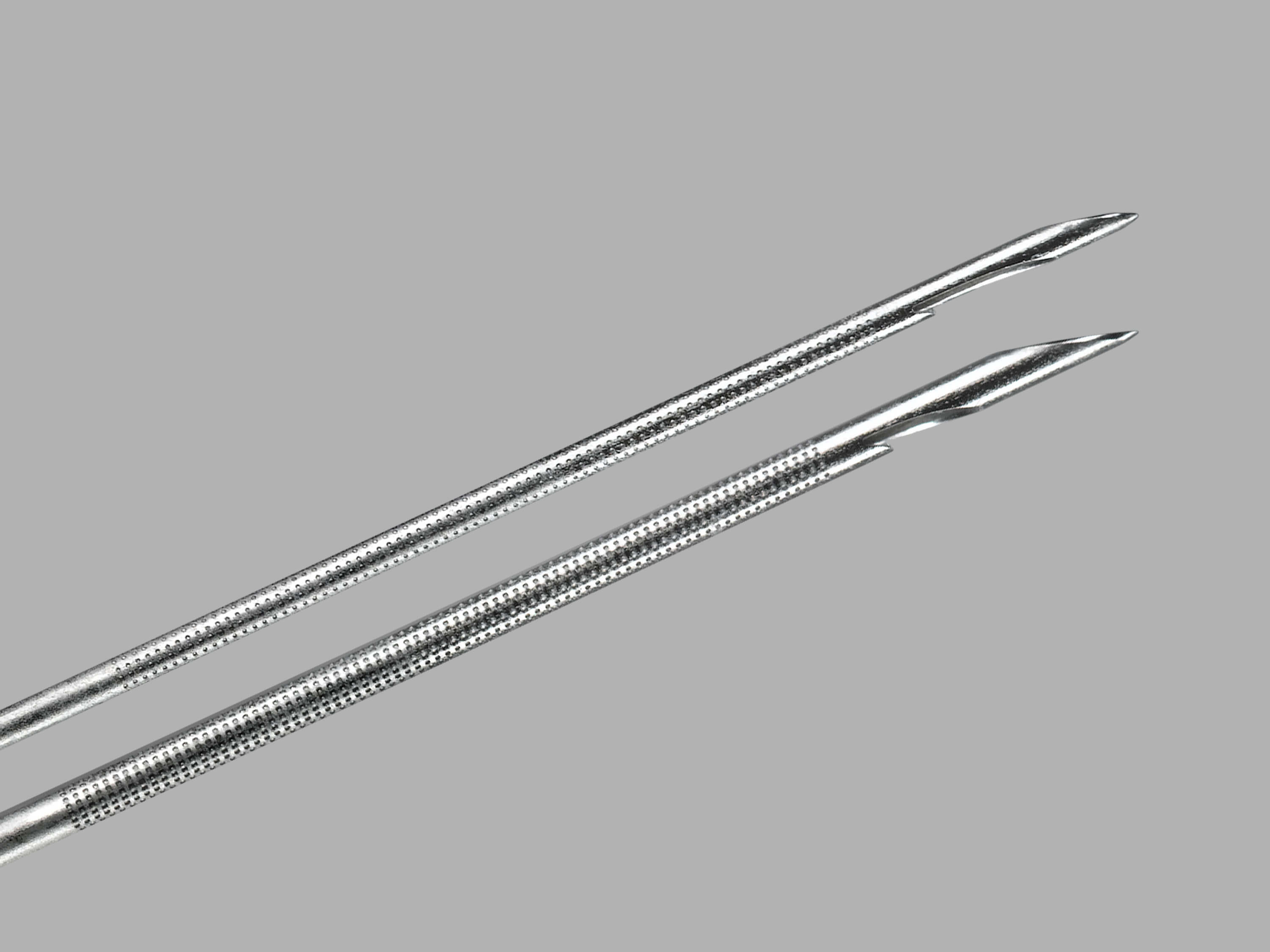
Bloomington, Ind.—Cook Medical is introducing the first endobronchial ultrasound (EBUS) needle in the U.S. and Europe that can acquire histological samples. The EchoTip® ProCore™ Endobronchial Ultrasound Needle gives physicians the ability to retrieve both cell and tissue samples from lymph…
February 4th, 2014
WEST LAFAYETTE, Ind. – Feb. 4, 2014 - Purdue Research Foundation and Cook Medical officials announced today plans to establish a $12 million evergreen investment fund to support Purdue-based life sciences companies. "Converting Purdue innovation into new products, companies and jobs…
December 11th, 2013
Winston-Salem, N.C.—Cook Medical has initiated a clinical study in the U.S. to evaluate the removability of a new Evolution® Esophageal Fully Covered Stent. This is the first multicenter U.S. study to evaluate the possibility of removing a self-expanding metal stent…
December 4th, 2013

Bloomington, Ind. – Mark Breedlove, a 19-year veteran of Cook Medical, has been named global leader for the company’s Peripheral Interventional (PI) division. “As our largest division, PI plays a vital role in Cook Medical’s global success,” said Pete Yonkman, president…
December 4th, 2013

Bloomington, Ind. – Rob Lyles, a veteran leader of Cook Medical’s Peripheral Intervention (PI) division, has been promoted to executive vice president in a move to further invigorate Cook’s drive toward new, cutting edge technologies and patient therapies. He will transition…
December 4th, 2013
Bloomington, Ind. – As hospitals adopt electronic commerce (e-commerce) as the standard for how they order products, Cook Medical is determined to improve the available e-commerce options for its customers. One recent improvement has been an enhancement of their online ordering…
November 13th, 2013

Bloomington, Ind. – Kem Hawkins, president of Cook Group Incorporated, announced today that Pete Yonkman will lead Cook Group’s medical companies as president of Cook Medical. Hawkins will continue serving as president of Cook Group. “Cook is an amazing company run…
November 13th, 2013

Bloomington, Ind. — Cook Medical has today officially opened its new state-of-the-art research and development (R&D) Innovation Centre at its plant in Limerick, Ireland. The Innovation Centre together, with the expansion of cleanrooms, packaging, storage and other facilities, represents…
October 24th, 2013

New Orleans, La. – The 2013 Arthur D. Smith Endourology Lectureship has been awarded to Thomas Knoll, MD, Associate Professor of Urology at University Medical Center Mannheim and the head of the Department of Urology at the Sindelfingen Medical center of…
October 8th, 2013
Las Vegas, Nev. — Four-year data from the Zilver® PTX® Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease from Cook Medical presented today at the 2013 Vascular Interventional Advances (VIVA) meeting demonstrates 75 percent primary patency in the superficial femoral…
October 2nd, 2013
Bloomington, Ind.—Cook Medical today marks the one-year anniversary of the formal launch of its Otolaryngology/Head and Neck Surgery Division (OHNS). Since launching at the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) conference in 2012, the division has successfully developed…
October 1st, 2013

Bloomington, Ind. – Gretchen Gutman, a 20-year veteran of legislative and government policy, has joined Cook Group as vice president of public policy. “The health care industry is constantly facing changes and issues at all levels of government policy,” said Dan…
September 5th, 2013
Bloomington, Ind. —Cook Medical has a new device to simplify percutaneous nephrolithotomy (PCNL) procedures, during which physicians break up and remove large kidney stones, or can use it in the bladder to break up large bladder stones. LithAssist™ combines suction…
August 23rd, 2013
Bloomington, Ind. — Effective October 1, Cook Medical’s Zilver® PTX® Drug-Eluting Peripheral Stent qualifies for new-technology add-on payments under Medicare’s hospital inpatient prospective payment system. “This is great news for Medicare beneficiaries who suffer from peripheral arterial disease, and for U.S.…
August 22nd, 2013
Bloomington, Ind. – Nicky James, a 15-year veteran of Cook Medical, has been named vice president and global leader of Cook’s Aortic Intervention (AI) clinical division. Phil Nowell, who has held that position for five years, will return to his native…
August 8th, 2013
Bloomington, Ind. — Cook Medical has completed patient enrollment in a study evaluating a technique for achieving vascular access via below-the-knee arteries. The new access technique could be used in treating peripheral arterial disease (PAD), including patients with critical limb ischemia…
August 7th, 2013
Bloomington, Ind. – Cook Medical is again shipping its Zilver® PTX® Drug-Eluting Peripheral Stent to medical centers in the U.S., Japan, Europe and other major markets. The shipments follow a brief period of unavailability due to a voluntary recall by Cook…
July 29th, 2013
Bloomington, Ind. — Cook Medical has partnered with IU Health to evaluate a potential treatment for peripheral arterial disease (PAD), a vascular disease of the legs. The treatment involves the placement of a stent, which is a small, metal mesh tube…
July 19th, 2013
Chicago, Ill. – Cook Medical launched a new treatment option today for rhinologists (nose and sinus specialists) who treat patients that suffer from difficult-to-heal conditions in the nasal passages. The Biodesign® ENT Repair Graft, which acts as an adjunct to aid…
June 19th, 2013
Bloomington, Ind. — Cook Medical is making its Hercules® 3 Stage Wire Guided Esophageal Balloon available to otolaryngologists to treat patients with gastrointestinal (GI) strictures. The device will be offered through Cook’s Otolaryngology-Head and Neck Surgery (OHNS) clinical division, while also…
June 7th, 2013
Bloomington, Ind. – In an effort to provide surgeons and nurses with an advanced tool to better monitor blood flow during free flap procedures, Cook Medical has launched the Doppler DP-M350 Blood Flow Monitor, a medical device at the forefront of…
June 5th, 2013
Winston-Salem, N.C. – The Food and Drug Administration has granted 510(k) clearance for the Evolution® Biliary Controlled-Release Uncovered Stent from Cook Medical. The biliary stent adds to Cook’s line of Evolution controlled-release stents for the gastrointestinal (GI) tract. It is the…
May 30th, 2013
Bloomington, Ind. — Cook Medical is one of several Indiana life science companies, three research universities and the state government helping launch a unique, industry-led biosciences research center in Indiana. The Indiana Biosciences Research Institute (IBRI), which was announced today at…
May 7th, 2013
San Diego, Cal.— At the American Urological Association’s (AUA) 2nd Annual National Residents Bowl, the country’s best and brightest residents competed in a three day battle of urology industry knowledge. Each competitor showed impressive knowledge about the history of urology,…
April 2nd, 2013
Bloomington, Ind.— Cook Group officials today acknowledged an Indiana General Assembly concurrent resolution sponsored by State Representative Bob Heaton extending "extreme gratitude" for the company’s 50-year history and its beneficial impact on Indiana’s economy and culture. "On behalf of the Cook family,…
March 25th, 2013
Bloomington, Ind. — Cook Pharmica, which develops and manufactures pharmaceutical and biopharmaceutical products on a contract basis, recently received more good news from the Food and Drug Administration (FDA). The company earned another commercial approval from the FDA and did…
March 22nd, 2013
Bloomington, Ind. — Cook Medical is gratified and U.S. patients are thankful for the bipartisan vote in the U.S. Senate on Thursday that overwhelmingly passed the Hatch-Klobuchar amendment to the Senate Budget Resolution to repeal the 2.3 percent tax on medical…
March 18th, 2013
Bloomington, Ind.— Cook Medical has launched a suite of salivary duct access products that offer minimally invasive options for the treatment of obstructive salivary gland disease. Minimally invasive treatment of obstructive salivary gland disease can reduce the need for invasive…
January 28th, 2013
Bloomington, Ind. — The only FDA approved ureteral access sheath that has two options for placement is now available in the United States. The Flexor©Parallel™ Rapid Release™ Ureteral Access Sheath allows urologists to use a single wire guide that functions as…
January 2nd, 2013
Bloomington, Ind. — The health care industry is adopting a set of standards to make supply chain easier for hospitals and Cook Medical is ready for the change. Almost ten years ago, Cook Medical realized all of its manufacturing companies were…
December 18th, 2012
Bloomington, Ind. — Just weeks after the Food and Drug Administration (FDA) approved Cook Medical’s Zilver© PTX© Drug-Eluting Peripheral Stent, Riverside Methodist Hospital in Columbus, Ohio, has treated the first patient with the device as part of Cook’s U.S. commercial…
November 15th, 2012
Bloomington, Ind. — Reinforcing its commitment to advancing interventional techniques and technologies to treat disease, Cook Medical is pleased to announce a new contract with leading health care supply chain and contracting company Novation for Cook’s peripheral intervention and peripheral diagnostic…
November 15th, 2012
Bloomington, Ind. — Cook Medical has received U.S. Food and Drug Administration (FDA) marketing approval for the first devices in its Zilver® PTX® Drug-Eluting Peripheral Stent portfolio, company officials reported today. It’s the first time the FDA has approved a drug-eluting…
November 1st, 2012
Bloomington, Ind. — New recommendations from the Association of Clinical Researchers and Educators (ACRE) to guide relationships between physicians and industry could pave the way for a new era of medical advances and bring waves of breakthrough devices to patients worldwide.…
October 22nd, 2012
Bloomington, Ind. — The Grant Street Inn has opened a new 16-room addition. The new building was designed and constructed to meet Leadership in Energy and Environmental Design (LEED) certification requirements. The facility will be the fourth LEED-certified building in Bloomington,…
October 22nd, 2012
Poway, Calif. — K-Tube Technologies is now working with two companies that will provide sales support and technical knowledge to customers. This collaboration with Medical Engineering Resources (MER) in Europe and Medical Metals in the United States will help all three…
October 12th, 2012
Las Vegas, Nevada — Three-year data from the Zilver® PTX® Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease from Cook Medical demonstrate 70.7 percent primary patency in the superficial femoral artery (SFA) at 36 months for patients treated with the…
October 5th, 2012
Bloomington, Ind. — Following Health Canada approval, Cook Medical made the Zilver Vena Venous Self-Expanding Stent available to physicians across Canada at the 2012 Annual Meeting of the Canadian Society for Vascular Surgery. Designed to restore blood flow in obstructed iliofemoral…
September 28th, 2012
Baesweiler, North Rhine-Westphalia — Cook Medical, a world leader in minimally invasive medical technologies, today announced a major development in the extension of its European operations with the opening of a new €15m distribution center in Baesweiler, Germany. Located less than…
September 19th, 2012
Washington, D.C. — As part of its commitment to advancing research in the field of otolaryngology/head and neck surgery (OHNS), Cook Medical with a strong history of philanthropy to encourage and support research and education, has supported the American Academy…
September 17th, 2012
Bloomington, Ind. — Victor Vinci, Ph.D., has joined Cook Pharmica's leadership team as vice president and chief scientific officer. Vinci will be responsible for guiding the scientific direction of Cook Pharmica, applying his more than 20 years of broad technical,…
September 10th, 2012
Washington, D.C. — Cook Medical, a world leader in minimally invasive medical device technology, has launched its new Otolaryngology/Head and Neck Surgery (OHNS) clinical division to bring the benefits of the company’s devices for non-surgical procedures to a new group…
September 6th, 2012
Istanbul, Turkey — The 2012 Arthur D. Smith Endourology Lectureship has been awarded to Mihir Mahesh Desai, M.D., D.N.B., director of the Center for Advanced Robotics at the University of Southern California (USC) Institute of Urology. Desai won the award,…
August 31st, 2012
Canton, IL — In a ceremony opening the company’s latest U.S. manufacturing facility, Cook Medical President Kem Hawkins joined national and local dignitaries today to dedicate a $19 million production plant that will add another 60 high-skill jobs to the…
August 27th, 2012
Pittsburgh, Pa. — Cook MyoSite Incorporated is expanding its workforce to more than double its current size by 2015. By the end of 2012 alone, Cook MyoSite plans to hire 12-15 new employees. Cook MyoSite is a part of the…
August 13th, 2012
Bloomington, Ind. — Previously unreleased three-year data from the Zilver PTX Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease indicate that Cook Medical's paclitaxel-eluting peripheral vascular stent demonstrated 83.0 percent freedom from TLR at 36 months in the PTX…
July 25th, 2012
Bloomington, Ind. — Reinforcing its commitment to advancing the field of interventional radiology (IR), Cook Medical today announced general availability of the Aprima Access Nonvascular Introducer Set, the first product in the Aprima drainage portfolio. It is a nonvascular access set…
July 9th, 2012
Winston-Salem, N.C. — The U.S. Food and Drug Administration (FDA) has granted Cook Medical 510(k) clearance for the Evolution® Colonic Controlled-Release Stent, Cook announced today. The new stent expands Cook's line of Evolution controlled-release stents and is used to palliate uncomfortable symptoms…
July 2nd, 2012
Solsberry, Ind. – CFC Properties announces the restoration of the Yoho General Store, with plans to reopen in the fall. The Solsberry, Ind. historic landmark was purchased by CFC in May. “The Yoho General Store has been a staple in…
June 28th, 2012
Bloomington, Ind. – With the U.S. Supreme Court ruling upholding the constitutionality of the Affordable Care Act (ACA), Cook Medical now calls for the U.S. Senate to repeal the 2.3 percent medical device excise tax included in that legislation. “The…
June 25th, 2012
Bloomington, Ind. – Clinical investigators are for the first time examining the retrograde tibiopedal interventional approach, an endovascular technique that has the potential to reduce the rate of leg amputations by as much as 50 percent1 in patients with critical limb ischemia…
June 18th, 2012
BLOOMINGTON, Ind. – In a decision that paves the way for greater pharmaceutical industry use of the company’s contract manufacturing services, Cook Pharmica LLC has received its first approval from the U.S. Food and Drug Administration (FDA) to manufacture commercial…
June 4th, 2012
Bloomington, Ind. – This is an important week for employees and businesses across Indiana and our nation. The Committee on Ways and Means in the U.S. House of Representatives, with support from both parties, voted 23-11 to approve legislation, H.R.…
May 21st, 2012
Bloomington, Ind. — Cook Medical, a world leader in minimally invasive medical technologies, has launched a first-of-its-kind drug-eluting peripheral stent in Australia, the Zilver® PTX® Drug-Eluting Peripheral Stent. The device, which is available in more than 50 countries, is registered…
May 21st, 2012
Bloomington, Ind. — Cook Medical has added a 25 gage needle to its EchoTip® ProCore™ line of fine needle biopsy (FNB) histology needles, which are designed for use in a procedure known as endoscopic ultrasound, or EUS. The EchoTip ProCore is…
May 21st, 2012
Poway, Calif. — K-Tube Technologies, the largest producer of miniature stainless steel tubing in the United States, is pleased to announce its new R&D process for device engineers, K-Tube Discover. Created for engineers in the early phases of design and…
May 1st, 2012
Bloomington, Ind. — In a development that brings advanced combination therapy treatment of peripheral artery disease (PAD) to Japanese patients for the first time, Cook Medical has received PMDA approval to sell the Zilver® PTX® Drug-Eluting Peripheral Stent in Japan.…
April 9th, 2012
Bloomington, Ind. — As part of its ongoing commitment to patients needing emerging new cellular therapies, Cook Group has acquired the assets of General BioTechnology LLC and launched a new company, Cook General BioTechnology LLC (CGBT). Based in Indianapolis, CGBT…
March 26th, 2012
San Francisco, Calif. — Cook Medical is pleased to announce the introduction of two bone biopsy products, the Osteo-Site® Ratchet™ and the Osteo-Site® coaxial bone biopsy needle set, at the Society of Interventional Radiology's (SIR) 37th Annual Scientific Meeting in…
February 1st, 2012
Dublin, Ohio — Cardinal Health and Cook Medical today announced a two-year, exclusive agreement for the North American distribution of Cook Medical central venous catheter (CVC) sets with Cardinal Health Presource® customizable procedural kits – providing clinicians with advanced technology…
January 23rd, 2012
Bloomington, Ind. — Hospitals around the country have three more years of access to a specialty urology agreement with an extensive line of specialty urology products at special pricing after Cook Medical and the Premier healthcare alliance renewed their contract…
January 16th, 2012
Cook Japan Incorporated and Medico's Hirata announce their joint commitment to restructure their medical device distribution route as of April 1st 2012 in order to provide more specialized support to Japanese subdealers, medical institutions and physicians. As of the beginning…
January 5th, 2012
Dublin – Minister for Jobs, Enterprise and Innovation Richard Bruton TD today announced that Cook Medical, the largest privately owned medical device company in the world, is to invest up to €16.5m over four years creating highly skilled positions in…
December 5th, 2011
Kyoto, Japan – Matthew T. Gettman, M.D., program director of the Mayo Clinic's Department of Urology, has been awarded the 2011 Arthur D. Smith Endourology Lectureship. The award, known as the “Arthur” award, was given at the 29th World Congress…
December 5th, 2011
Bloomington, Ind. – Beginning January 1, 2012, Cook Medical’s central venous catheters (CVCs) will be available to the members served by Novation, the supply contracting company for more than 30,000 members of VHA Inc., UHC, and Provista. This expands nationwide…
October 28th, 2011
Limerick, Ireland – Cook Medical's valuable place among the business community in Limerick has been recognised by Limerick Chamber at the inaugural Limerick Region Business Awards with Cook Ireland winning Company of the Year and Best Foreign Direct Investment Company…
October 17th, 2011
Cook Medical announced today that its Evolution® Duodenal Controlled-Release Stent was recently granted 510(k) clearance by the Food & Drug Administration (FDA). This new stent expands palliative care options for patients experiencing issues associated with malignant gastric outlet obstruction (GOO),…
September 19th, 2011
Bloomington, IN – Cook Medical will present data supporting the submission for its Zilver® PTX® Drug-Eluting Stent in the superficial femoral artery (SFA) to the Food and Drug Administration’s (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee on…
September 19th, 2011
Bloomington, IN – Cook Medical will present data supporting the submission for its Zilver® PTX® Drug-Eluting Stent in the superficial femoral artery (SFA) to the Food and Drug Administration’s (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee…
September 9th, 2011
Bloomington, Ind. – On the heels of President Obama’s speech to Congress about the critical need to accelerate US job growth, Cook Medical today applauded a new study from AdvaMed demonstrating that a new medical device excise tax would cost the…
September 9th, 2011
Munich, Germany – Cook Medical, a world leader in minimally invasive medical technologies, has launched the world’s first-ever stent designed and approved specifically to treat symptomatic iliofemoral venous outflow obstruction. The Zilver Vena Venous Self-Expanding Stent has received CE Mark…
September 7th, 2011
Bloomington, Ind. -- Cook Medical supports the FDA's efforts to protect women's health and its review of the issues surrounding the use of surgical mesh for the treatment of pelvic floor disorders. We commend the FDA's support of open scientific…
September 1st, 2011
Bloomington, Ind. — Urologists around the globe will soon have access to an interactive and collaborative training program, as Cook Medical officials announced the launch of their Urology division’s physician education program, a part of a company wide Vista Collaboration…
August 19th, 2011
Bloomington, Ind. – Cook Incorporated, a global leader in endovascular technologies, announced today that Judge Tanya Walton Pratt of the United States District Court for the Southern District of Indiana in Indianapolis has issued a Markman ruling in the ongoing…
August 8th, 2011
Bloomington, Ind. – Physicians will now have access to Cook Medical’s Word catheter, a silicone balloon catheter used to treat cysts of the Bartholin gland, company officials announced today. Cysts in the Bartholin gland occur when the gland becomes blocked,…
July 14th, 2011
Bloomington, Ind. – Cook Medical applauds the FDA’s efforts to protect women’s health by continuing to investigate the issues surrounding the use of surgical mesh for the treatment of pelvic floor disorders. We commend the FDA’s support of open scientific…
July 14th, 2011
Bloomington, Ind. -- Continually striving to enhance patient care, Cook Medical recently announced significant updates to its Resonance Metallic Ureteral Stent System. First launched in 2007, the device now has an updated introducer system that includes a clear sheath for…
July 12th, 2011
Bloomington, Ind. — Cook Medical has received premarket approval (PMA) from the U.S. Food and Drug Administration (FDA) for its Zenith® Spiral-Z™ AAA Iliac Leg Graft. The device is indicated for use in patients with abdominal aortic aneurysms (AAA) that…
June 27th, 2011
June 27, 2011 Washington, DC — Two Cook Pharmica executives will speak this week at the 2011 BIO International Convention: The Global Event for Biotechnology held June 27-30 in Washington, D.C. Ryan Hawkins and E. Morrey Atkinson will discuss two…
June 27th, 2011
Shanghai, China – Cook Medical, one of the world’s leading developers of health care devices, is expanding the company’s first Asia-Pacific Distribution Centre (APDC) to meet the growing demand for medical equipment throughout the region, company officials report. Cook Medical…
June 22nd, 2011
Warwickshire -- Cook Medical, a world leader in the development of advanced endovascular devices treating aortic disease, has sponsored a medical education programme created and administered by the British Society of Endovascular Therapy (BSET) to train British physicians in advanced…
June 7th, 2011
Bloomington, Ind. –Results from a multicenter European study1 suggest that a new, large-core biopsy needle designed for use with an endoscopic ultrasound (EUS) scope may help to overcome limitations of current EUS methods for biopsies of lesions in the gastrointestinal…
May 18th, 2011
Bloomington, Ind. May 18, 2011 – Patients in more than 54,000 health care facilities will gain access to Cook Medical's full line of peripherally inserted central venous catheters (PICCs), including the Cook Spectrum® Turbo-Ject® antibiotic-impregnated PICC, effective May 1, 2011.…
May 9th, 2011
Canton, Ill. – Cook Polymer Technology, a raw materials manufacturer and part of Cook Group, will expand its operations by opening a new plant in Canton, Ill., company officials announced at a ground-breaking ceremony today. This project, with costs estimated…
May 9th, 2011
Chicago, Ill. – An increasingly popular technique for removing lesions associated with Barrett’s esophagus has been deemed a safe and effective treatment option in a study of more than 1,000 resections published this month in the European journal Endoscopy.¹ The…
May 4th, 2011
San Francisco, Calif. — A new version of the Liberator Locking Stylet with Beacon® Tip Technology, a distinct radiopaque tip marker, was unveiled at Heart Rhythm 2011, the 32nd Annual Scientific Sessions of the Heart Rhythm Society, and is now…
April 15th, 2011
Bloomington, Ind. – William Alfred Cook, founder of the Cook Group global network of companies and a pioneer in the development of life-saving minimally invasive medical device technology, died Friday at approximately 4:30 p.m. EDT at his Bloomington home of congestive…
March 28th, 2011
Bloomington, Ind. – Cook Medical invited urologists to sign up for the Resonance® Registry at the European Association of Urology Annual Congress which took place in Vienna, Austria, earlier this week. The registry has been set up in order to…
March 24th, 2011
Bloomington, Ind. - Dan Sirota, who has served as global business leader for Cook Medical’s Interventional Radiology division since its inception in 2009, has been promoted to vice president of that business unit, the company said today. As vice president,…
March 21st, 2011
Bloomington, Ind. – Cory Lewis has been promoted to vice president of business development and marketing, Cook Pharmica announced recently. In his new role, Lewis will lead business development, marketing and project management functions. “Cory’s passion for creating positive customer…
March 21st, 2011
Bloomington, Ind. – Ryan Hawkins has been promoted to vice president of drug product operations, Cook Pharmica officials announced recently. In his new role, Hawkins will continue to lead all aspects of the drug product business unit, with an increased…
March 21st, 2011
Bloomington, Ind. – Steve Perry has been promoted to vice president of drug substance operations, Cook Pharmica announced recently. Perry will continue to lead the drug substance business unit and will assume responsibility for the engineering function in his new…
February 24th, 2011
Bloomington, Ind. – Joe Roberts, a veteran of the Cook organization, has been named vice president of corporate development, Cook Medical officials announced today. Roberts will report to Jerry Williams, senior vice president of corporate development, and will be responsible…
February 16th, 2011
WINSTON-SALEM, N.C. — A new histology needle for endoscopic ultrasound (EUS) from Cook Medical is now on the market, giving physicians the ability to retrieve tissue samples from hard-to-reach regions within or adjacent to the GI tract with a minimally…
February 15th, 2011
Bloomington, Ind. – As part of its continuing effort to provide cardiovascular health, family fitness education and leadership opportunities to residents of Monroe County, the Monroe County YMCA announced today the start of the “Strong Past, Bright Future” fundraising campaign…
January 31st, 2011
Bloomington, Ind. – E. Morrey Atkinson, Ph.D., has joined Cook Pharmica as vice president of research and development and chief scientific officer, company officials announced recently. Atkinson will be responsible for guiding the scientific direction of Cook Pharmica, relying on…
January 31st, 2011
Bloomington, Ind. – Patients at more than 2,400 hospitals and 70,000 health care facilities will have access to Cook Medical’s line of diagnostic and interventional radiology products, including peripheral vascular and biliary stents, effective February 1, 2011. Cook announced today…
January 18th, 2011
Bloomington, Ind. — Cook Medical has received premarket approval (PMA) from the U.S. Food and Drug Administration for its Formula™ Balloon-Expandable Renal Stent System. The approval includes both the Formula 414RX Balloon Expandable Renal Stent and the Formula 418 Balloon…
January 17th, 2011
Miami, Fla. – An investigational drug-eluting stent (DES) from Cook Medical showed sustained primary patency at two years compared to data collected at one year in the device’s prospective, randomized study, according to data presented today at the ISET 2010…
January 17th, 2011
Bloomington, Ind. – Study investigators today reported initial data from Cook Medical’s REFORM clinical trial that is aimed at assessing the safety and effectiveness of the company’s balloon-expandable renal stent for the treatment of renal artery stenosis. The data, presented…
 Bloomington, Ind. — Cook Medical announced this morning it has signed a letter of intent with Astorg, a leading private equity firm with an extensive and successful track record in global healthcare investments, to purchase Cook’s Reproductive Health business (“Cook…
Bloomington, Ind. — Cook Medical announced this morning it has signed a letter of intent with Astorg, a leading private equity firm with an extensive and successful track record in global healthcare investments, to purchase Cook’s Reproductive Health business (“Cook…  Bloomington, Ind. — Cook Medical announced this morning it has signed a letter of intent with Astorg, a leading private equity firm with an extensive and successful track record in global healthcare investments, to purchase Cook’s Reproductive Health business (“Cook…
Bloomington, Ind. — Cook Medical announced this morning it has signed a letter of intent with Astorg, a leading private equity firm with an extensive and successful track record in global healthcare investments, to purchase Cook’s Reproductive Health business (“Cook…  Bloomington, Ind. — Ross Whittaker, a manager of manufacturing engineering at Cook, was named a member of the Conexus Rising 30 Class of 2024 for his exceptional work in advanced manufacturing and logistics (AML) and the changes he and his…
Bloomington, Ind. — Ross Whittaker, a manager of manufacturing engineering at Cook, was named a member of the Conexus Rising 30 Class of 2024 for his exceptional work in advanced manufacturing and logistics (AML) and the changes he and his…  At Cook, our goal has always been to provide devices and services to support minimally invasive procedures for patients. That hasn’t changed. However, what has changed is the way we work toward that goal. In November 2023, we announced an…
At Cook, our goal has always been to provide devices and services to support minimally invasive procedures for patients. That hasn’t changed. However, what has changed is the way we work toward that goal. In November 2023, we announced an…  Bloomington, Ind. — In her more than 15 years of experience at Cook Medical, Amy Tyrrell has always prioritized relationships—both inside and outside the company. Her hard work over the years was recognized when she earned the Captis Medical Device…
Bloomington, Ind. — In her more than 15 years of experience at Cook Medical, Amy Tyrrell has always prioritized relationships—both inside and outside the company. Her hard work over the years was recognized when she earned the Captis Medical Device…  Cook Medical has released its Social Impact & Sustainability Report for 2023, highlighting the company’s commitment to working sustainably and responsibly across all of its businesses while fulfilling its purpose to improve the lives of everyone it serves. The report…
Cook Medical has released its Social Impact & Sustainability Report for 2023, highlighting the company’s commitment to working sustainably and responsibly across all of its businesses while fulfilling its purpose to improve the lives of everyone it serves. The report…  Cook Medical’s Beacon® Tip Sizing Catheters are now available in the US and Canada. The Beacon Tip Sizing Catheter is available in a variety of lengths and tip configurations, empowering physicians to treat patients with the best fitting devices for…
Cook Medical’s Beacon® Tip Sizing Catheters are now available in the US and Canada. The Beacon Tip Sizing Catheter is available in a variety of lengths and tip configurations, empowering physicians to treat patients with the best fitting devices for…  An Interview with Cook chief medical officer Dr. John Kaufman About 20 years ago, Cook started working on a venous valve. Our goal was to create a long-term solution for patients suffering from chronic venous insufficiency (CVI) due to reflux…
An Interview with Cook chief medical officer Dr. John Kaufman About 20 years ago, Cook started working on a venous valve. Our goal was to create a long-term solution for patients suffering from chronic venous insufficiency (CVI) due to reflux…  Bloomington, Ind. — Cook Medical and EnteraSense, Ltd. have entered into an agreement where Cook will become the exclusive distributor of EnteraSense’s…
Bloomington, Ind. — Cook Medical and EnteraSense, Ltd. have entered into an agreement where Cook will become the exclusive distributor of EnteraSense’s…  Bloomington, Ind. — Cook Medical launched the next-generation EchoTip AcuCore™ Endoscopic Ultrasound (EUS) Biopsy Needle in the United States. As Cook changes to better serve our customers, we look forward to developing and offering products for a wider variety of…
Bloomington, Ind. — Cook Medical launched the next-generation EchoTip AcuCore™ Endoscopic Ultrasound (EUS) Biopsy Needle in the United States. As Cook changes to better serve our customers, we look forward to developing and offering products for a wider variety of…  Bloomington, Ind. — Cook Medical is now offering the Cook Medical's Ascend™ Single-Use Flexible Ureteroscope in the United States and Canada. This addition is a positive change that will empower Cook to better serve more urology customers with a complete…
Bloomington, Ind. — Cook Medical is now offering the Cook Medical's Ascend™ Single-Use Flexible Ureteroscope in the United States and Canada. This addition is a positive change that will empower Cook to better serve more urology customers with a complete…  Bloomington, Ind. — Cook Medical has been awarded an ECAT contract with the United States Department of Defense for implantable medical devices. This contract is one way Cook is being recognized for the ways we are changing to treat more…
Bloomington, Ind. — Cook Medical has been awarded an ECAT contract with the United States Department of Defense for implantable medical devices. This contract is one way Cook is being recognized for the ways we are changing to treat more…  Bloomington, Ind. — While Marsha Lovejoy has been leading public relations at Cook Medical, she’s also been dedicating her time to community service and advocacy and…
Bloomington, Ind. — While Marsha Lovejoy has been leading public relations at Cook Medical, she’s also been dedicating her time to community service and advocacy and…  Bloomington, IN and Merrimack, NH—Today, Getinge and Cook Medical announced an exclusive sales and distribution agreement for the iCast® covered stent system, which recently received premarket approval for treatment of symptomatic iliac arterial occlusive disease. Cook Medical will assume…
Bloomington, IN and Merrimack, NH—Today, Getinge and Cook Medical announced an exclusive sales and distribution agreement for the iCast® covered stent system, which recently received premarket approval for treatment of symptomatic iliac arterial occlusive disease. Cook Medical will assume…  Bloomington, Ind. — Today, Cook Medical and Bentley announced a United States distribution agreement for the BeBack Catheter. Over the coming months, Cook Medical will be assuming commercial responsibilities for this Bentley product. “We are excited to welcome Bentley’s BeBack…
Bloomington, Ind. — Today, Cook Medical and Bentley announced a United States distribution agreement for the BeBack Catheter. Over the coming months, Cook Medical will be assuming commercial responsibilities for this Bentley product. “We are excited to welcome Bentley’s BeBack…  Bloomington, Ind. — Cook Medical’s shorter Liver Access and Biopsy Set (LABS) is back on the US market with a new pediatric indication. Originally indicated for use in adults only, the set now has FDA clearance for use in adolescents,…
Bloomington, Ind. — Cook Medical’s shorter Liver Access and Biopsy Set (LABS) is back on the US market with a new pediatric indication. Originally indicated for use in adults only, the set now has FDA clearance for use in adolescents,…  Bloomington, Ind. and Belgium — Cook Medical, a global medical device manufacturer, has partnered with Bedal International, a company specializing in catheter securement devices marketed under the brand FlexGRIP®. FlexGRIP devices are now available as an addition to Cook’s comprehensive…
Bloomington, Ind. and Belgium — Cook Medical, a global medical device manufacturer, has partnered with Bedal International, a company specializing in catheter securement devices marketed under the brand FlexGRIP®. FlexGRIP devices are now available as an addition to Cook’s comprehensive…  Bloomington, Ind. — Cook Medical’s Motion Hybrid Wire Guide is now commercially available in Canada. This two-in-one wire guide is an excellent choice for clinicians in urological specialties because it functions as both an access wire guide and a safety…
Bloomington, Ind. — Cook Medical’s Motion Hybrid Wire Guide is now commercially available in Canada. This two-in-one wire guide is an excellent choice for clinicians in urological specialties because it functions as both an access wire guide and a safety…  CAUTION - INVESTIGATIONAL DEVICE. Limited by United States law to investigational use. Bloomington, Ind. — Cook Medical today announced the first patient treated in the clinical study of the ZENITH® FENESTRATED+ Endovascular Graft (ZFEN+) in the United States. The procedure was performed…
CAUTION - INVESTIGATIONAL DEVICE. Limited by United States law to investigational use. Bloomington, Ind. — Cook Medical today announced the first patient treated in the clinical study of the ZENITH® FENESTRATED+ Endovascular Graft (ZFEN+) in the United States. The procedure was performed…  Bloomington, Ind. — Cook Medical today announced the sale of the remaining Otolaryngology, Head & Neck Surgery (OHNS) product lines, in the latest of a series of strategic decisions aligned to its 5-year vision. Cook signed an agreement with C2Dx,…
Bloomington, Ind. — Cook Medical today announced the sale of the remaining Otolaryngology, Head & Neck Surgery (OHNS) product lines, in the latest of a series of strategic decisions aligned to its 5-year vision. Cook signed an agreement with C2Dx,…  Bloomington, Ind. — Cook Medical’s Slip-Cath® Beacon® Tip Hydrophilic Selective Catheter is now available for use in the U.S. and Canada. Slip-Cath…
Bloomington, Ind. — Cook Medical’s Slip-Cath® Beacon® Tip Hydrophilic Selective Catheter is now available for use in the U.S. and Canada. Slip-Cath…  Bloomington, Ind. — Cook Medical won the 2023 Loyal Partner Supplier Award from Capstone Health Alliance for outstanding work from Cook’s MedSurg division. Cook is proud…
Bloomington, Ind. — Cook Medical won the 2023 Loyal Partner Supplier Award from Capstone Health Alliance for outstanding work from Cook’s MedSurg division. Cook is proud…  The last several years have brought significant change to Cook. To ensure that we are positioned to be successful in this new world, our global leadership teams have implemented a new vision and strategic plan that were informed by extensive…
The last several years have brought significant change to Cook. To ensure that we are positioned to be successful in this new world, our global leadership teams have implemented a new vision and strategic plan that were informed by extensive…  Bloomington, Ind. — The US Department of Veterans Affairs (VA) has awarded Cook Medical a Next Generation Implant Contract for implanted medical devices. This contract will streamline the procurement process for the VA supply chain team. This contract is another…
Bloomington, Ind. — The US Department of Veterans Affairs (VA) has awarded Cook Medical a Next Generation Implant Contract for implanted medical devices. This contract will streamline the procurement process for the VA supply chain team. This contract is another…  Bloomington, Ind. — Cook Medical is pleased to promote Barry Slowey to vice president and chief sustainability officer. His new position was effective September 8, 2023. In his new appointment, Barry will provide leadership and implement the goals of Cook’s Social…
Bloomington, Ind. — Cook Medical is pleased to promote Barry Slowey to vice president and chief sustainability officer. His new position was effective September 8, 2023. In his new appointment, Barry will provide leadership and implement the goals of Cook’s Social…  Bloomington, Ind. — At the 2023 Vizient Connections Summit, Cook Medical won the Vizient Supply Assurance Supplier of the Year Award. This award demonstrates Cook’s transparency and commitment to communication during the supply chain process with healthcare organizations that are…
Bloomington, Ind. — At the 2023 Vizient Connections Summit, Cook Medical won the Vizient Supply Assurance Supplier of the Year Award. This award demonstrates Cook’s transparency and commitment to communication during the supply chain process with healthcare organizations that are…  Bloomington, Ind. — Cook Medical is pleased to promote Amber Beauchamp to vice president, global human resources at Cook Group and Cook Medical effective September 1, 2023. Beauchamp has more than 17 years of HR experience in multiple industries. She…
Bloomington, Ind. — Cook Medical is pleased to promote Amber Beauchamp to vice president, global human resources at Cook Group and Cook Medical effective September 1, 2023. Beauchamp has more than 17 years of HR experience in multiple industries. She…  Bringing the latest in medical technologies to the youngest patients INDIANAPOLIS and WEST LAFAYETTE, Ind. – Purdue University, the Indiana University School of Medicine and medical device company Cook Medical are focusing a new alliance on clinical needs in pediatrics…
Bringing the latest in medical technologies to the youngest patients INDIANAPOLIS and WEST LAFAYETTE, Ind. – Purdue University, the Indiana University School of Medicine and medical device company Cook Medical are focusing a new alliance on clinical needs in pediatrics…  Bloomington, Ind. — As announced at the 2023 Vascular Annual Meeting (VAM), the Advance Serenity® Hydrophilic PTA Balloon Catheter product line is now available with even more options. Interventionalists who perform peripheral intervention procedures in the US and Canada now…
Bloomington, Ind. — As announced at the 2023 Vascular Annual Meeting (VAM), the Advance Serenity® Hydrophilic PTA Balloon Catheter product line is now available with even more options. Interventionalists who perform peripheral intervention procedures in the US and Canada now…  For five years, the Cook Medical headquarters in Bloomington, Indiana has hosted e-recycling events. We held it again this year, and the tradition has recently sparked other electronics recycling events at other Cook locations. It’s a part of who we…
For five years, the Cook Medical headquarters in Bloomington, Indiana has hosted e-recycling events. We held it again this year, and the tradition has recently sparked other electronics recycling events at other Cook locations. It’s a part of who we…  Bloomington, Ind. — Cook Medical is proud to achieve a transparency partner badge from the Healthcare Industry Resiliency Collaborative (HIRC). HIRC is a nonprofit healthcare supply chain trade association. Its mission is to support supply chain best practices for better…
Bloomington, Ind. — Cook Medical is proud to achieve a transparency partner badge from the Healthcare Industry Resiliency Collaborative (HIRC). HIRC is a nonprofit healthcare supply chain trade association. Its mission is to support supply chain best practices for better…  The email below, along with a video from our president and an FAQ document, was sent to all Cook Medical employees on Monday, May 15 at 1pm EST. Memo header: Company announcement: A difficult step for our strategic success…
The email below, along with a video from our president and an FAQ document, was sent to all Cook Medical employees on Monday, May 15 at 1pm EST. Memo header: Company announcement: A difficult step for our strategic success…  Bloomington, Ind. — For the third year in a row, Cook Medical has received a Greenovation award from Kimberly-Clark’s RightCycle Program. The award is for Cook’s continued sustainability leadership and participation in a landfill diversion program for nitrile gloves. [caption…
Bloomington, Ind. — For the third year in a row, Cook Medical has received a Greenovation award from Kimberly-Clark’s RightCycle Program. The award is for Cook’s continued sustainability leadership and participation in a landfill diversion program for nitrile gloves. [caption…  Jennifer Ludden and Marisa Peñaloza shared Cook Medical's housing initiative on NPR's Morning Edition. The piece is called "Would you live next to co-workers for the right price? This company is betting yes." NPR: Listen here On Tuesday, May 2,…
Jennifer Ludden and Marisa Peñaloza shared Cook Medical's housing initiative on NPR's Morning Edition. The piece is called "Would you live next to co-workers for the right price? This company is betting yes." NPR: Listen here On Tuesday, May 2,…  Bloomington, Ind. — Cook Medical announces the first patient treated in a clinical study to evaluate a new venous valve designed for treating chronic venous insufficiency. The patient was treated by Dr. Mauricio Alviar, vascular surgeon and principal investigator, of…
Bloomington, Ind. — Cook Medical announces the first patient treated in a clinical study to evaluate a new venous valve designed for treating chronic venous insufficiency. The patient was treated by Dr. Mauricio Alviar, vascular surgeon and principal investigator, of…  Bloomington, Ind. — Cook Medical has received a contract with Vizient for endoscopy devices. This new agreement will allow Cook to continue to offer our endoscopy devices at negotiated pricing terms to healthcare facilities that are members of Vizient. With…
Bloomington, Ind. — Cook Medical has received a contract with Vizient for endoscopy devices. This new agreement will allow Cook to continue to offer our endoscopy devices at negotiated pricing terms to healthcare facilities that are members of Vizient. With…  Cook Medical aims to raise awareness around head and neck cancer and dysphagia through Dale Maloney’s story According to the Yale School of Medicine, head and neck cancer affected over 46,000 people last year.1 In addition, a study conducted in…
Cook Medical aims to raise awareness around head and neck cancer and dysphagia through Dale Maloney’s story According to the Yale School of Medicine, head and neck cancer affected over 46,000 people last year.1 In addition, a study conducted in…  Meredith Hackler, a reporter with WRTV, recently shared a story about Cook Medical on WRTV’s Hiring Hoosiers called “Workforce pilot program will aim to hire more people with disabilities.” WRTV, a news station local to Indianapolis, featured a story on…
Meredith Hackler, a reporter with WRTV, recently shared a story about Cook Medical on WRTV’s Hiring Hoosiers called “Workforce pilot program will aim to hire more people with disabilities.” WRTV, a news station local to Indianapolis, featured a story on…  Solar arrays brighten a sustainable future at Cook’s headquarters They traveled by steam ship and by semi-trailer truck across the globe to settle in Bloomington, Indiana. The journey began in Kolkata, India. Over 3,000 solar panels were carefully bundled in…
Solar arrays brighten a sustainable future at Cook’s headquarters They traveled by steam ship and by semi-trailer truck across the globe to settle in Bloomington, Indiana. The journey began in Kolkata, India. Over 3,000 solar panels were carefully bundled in…  Bloomington, Ind. — Cook Medical has launched a new, streamlined portfolio of urological bipolar electrodes in the U.S. This portfolio includes the products that urologists use most frequently to focus on daily electrode needs when performing procedures on the bladder…
Bloomington, Ind. — Cook Medical has launched a new, streamlined portfolio of urological bipolar electrodes in the U.S. This portfolio includes the products that urologists use most frequently to focus on daily electrode needs when performing procedures on the bladder…  Erik L. helps Cook share knowledge with global decision-makers and thought leaders in the field of health economics Erik L. is the Health Economics manager for Cook in the United States, and his team is part of the Health Economics…
Erik L. helps Cook share knowledge with global decision-makers and thought leaders in the field of health economics Erik L. is the Health Economics manager for Cook in the United States, and his team is part of the Health Economics…  Raised to help others, Cook employee honors his mother in the most charitable way Jeff P. started at Cook Medical in 2014 as an Endoscopy district manager for the San Diego territory. After six years, he was promoted to Market…
Raised to help others, Cook employee honors his mother in the most charitable way Jeff P. started at Cook Medical in 2014 as an Endoscopy district manager for the San Diego territory. After six years, he was promoted to Market…  BLOOMINGTON, Ind. — It its inaugural HR Impact Awards, the Indianapolis Business Journal recognized Cook Medical’s High School Equivalency program. Cook’s program won in the Training and Development category of the HR Impact awards, a category that recognizes programs that help…
BLOOMINGTON, Ind. — It its inaugural HR Impact Awards, the Indianapolis Business Journal recognized Cook Medical’s High School Equivalency program. Cook’s program won in the Training and Development category of the HR Impact awards, a category that recognizes programs that help…  Davis P. and Alex B. already had a couple of things in common—they’re both Indianapolis natives and both work in Cook’s MedSurg Division. This spring they added a third thing to that list when it was announced that they had…
Davis P. and Alex B. already had a couple of things in common—they’re both Indianapolis natives and both work in Cook’s MedSurg Division. This spring they added a third thing to that list when it was announced that they had…  Bloomington, Ind. — About 3,000 solar panels now line the rooftops at Cook Medical’s headquarters. This project to invest in sustainable solar energy is Cook’s most recent initiative to create cleaner, healthier communities. At the beginning of 2022, Cook Medical…
Bloomington, Ind. — About 3,000 solar panels now line the rooftops at Cook Medical’s headquarters. This project to invest in sustainable solar energy is Cook’s most recent initiative to create cleaner, healthier communities. At the beginning of 2022, Cook Medical…  Bloomington, Ind. — Two Cook Medical managers were nominated for and selected as recipients of this year’s 23 Pair Awards hosted by BioCrossroads. Jillian Ivers and Lauren Manges lead teams at Cook and have been identified as outstanding emerging talents…
Bloomington, Ind. — Two Cook Medical managers were nominated for and selected as recipients of this year’s 23 Pair Awards hosted by BioCrossroads. Jillian Ivers and Lauren Manges lead teams at Cook and have been identified as outstanding emerging talents…  Indianapolis, Ind. – The Indy Fresh Market will have a one-time economic impact totaling $11.1 million, plus an annual impact of $4.6 million in wages and benefits, and related spending. An estimated 39 direct jobs and 61 full-time equivalent jobs will…
Indianapolis, Ind. – The Indy Fresh Market will have a one-time economic impact totaling $11.1 million, plus an annual impact of $4.6 million in wages and benefits, and related spending. An estimated 39 direct jobs and 61 full-time equivalent jobs will…  The lockdown in Shanghai and the employees who helped by moving in to the facility Editor's note: Shanghai has begun lifting a strict, two-month lockdown that was designed to contain rising Covid cases. We want to share an interesting…
The lockdown in Shanghai and the employees who helped by moving in to the facility Editor's note: Shanghai has begun lifting a strict, two-month lockdown that was designed to contain rising Covid cases. We want to share an interesting…  Artist Amiah Mims brings murals to life at the 38th and Sheridan facility Editor’s note: We believe that it’s possible to do good business while doing good in our communities. We’re proud to work in collaboration with partners on a…
Artist Amiah Mims brings murals to life at the 38th and Sheridan facility Editor’s note: We believe that it’s possible to do good business while doing good in our communities. We’re proud to work in collaboration with partners on a…  Bloomington, Ind. — Cook Medical has received a Chelsea Santucci Greenovation Award from Kimberly-Clark Professional. Cook won this award for its industry-leading efforts in recycling nitrile gloves. Cook Medical was recognized for participating in The RightCycle Program, the first large-scale…
Bloomington, Ind. — Cook Medical has received a Chelsea Santucci Greenovation Award from Kimberly-Clark Professional. Cook won this award for its industry-leading efforts in recycling nitrile gloves. Cook Medical was recognized for participating in The RightCycle Program, the first large-scale…  Bloomington, Ind. — The MINC+™ Benchtop Incubator is now available to clinical embryologists and IVF clinics in the US and Canada. The MINC+ is the next generation of the MINC® Mini Incubator, a benchtop incubator that has been a standard…
Bloomington, Ind. — The MINC+™ Benchtop Incubator is now available to clinical embryologists and IVF clinics in the US and Canada. The MINC+ is the next generation of the MINC® Mini Incubator, a benchtop incubator that has been a standard…  Bloomington, Ind. — Two Cook Medical employees applied and were selected for a fellowship from the Mitch Daniels Leadership Foundation (MDLF). Davis Payton and Alex Brethauer, both managers at Cook Medical, have shown exceptional commitment to innovation and investment in…
Bloomington, Ind. — Two Cook Medical employees applied and were selected for a fellowship from the Mitch Daniels Leadership Foundation (MDLF). Davis Payton and Alex Brethauer, both managers at Cook Medical, have shown exceptional commitment to innovation and investment in…  Today’s ribbon cutting ceremony marks a new beginning in a community that was once forgotten. Indianapolis, Ind. — A unique partnership between neighbors, a medical device company, government and non-profits today marks an important milestone today at 38th and Sheridan…
Today’s ribbon cutting ceremony marks a new beginning in a community that was once forgotten. Indianapolis, Ind. — A unique partnership between neighbors, a medical device company, government and non-profits today marks an important milestone today at 38th and Sheridan…  There was no easing into their workday for Susan S. and the rest of the nursing staff in the COVID-19 intensive care unit (ICU) at their hospital in Copenhagen, Denmark. At the beginning of every shift, they would gather to…
There was no easing into their workday for Susan S. and the rest of the nursing staff in the COVID-19 intensive care unit (ICU) at their hospital in Copenhagen, Denmark. At the beginning of every shift, they would gather to…  Winston-Salem, N.C. — Cook Medical has always been committed to improving people’s lives. That promise spans from patient care to the communities in which the company operates and beyond. Today Cook Medical’s Winston-Salem manufacturing facility announced that they have recently…
Winston-Salem, N.C. — Cook Medical has always been committed to improving people’s lives. That promise spans from patient care to the communities in which the company operates and beyond. Today Cook Medical’s Winston-Salem manufacturing facility announced that they have recently…  View news coverage of this announcement below. BLOOMINGTON, Ind. — Intended to serve middle-income workers, workforce housing is in short supply in communities across south-central Indiana, especially during the current housing price spike. After hearing from employees and surrounding communities, Cook…
View news coverage of this announcement below. BLOOMINGTON, Ind. — Intended to serve middle-income workers, workforce housing is in short supply in communities across south-central Indiana, especially during the current housing price spike. After hearing from employees and surrounding communities, Cook…  Bloomington, Ind. — Cook Medical’s Zenith® Thoraco+ Endovascular System (Thoraco+) has received Breakthrough Device Designation from the US Food and Drug Administration (FDA). This designation is granted to devices that have the potential to provide more effective treatment or diagnosis…
Bloomington, Ind. — Cook Medical’s Zenith® Thoraco+ Endovascular System (Thoraco+) has received Breakthrough Device Designation from the US Food and Drug Administration (FDA). This designation is granted to devices that have the potential to provide more effective treatment or diagnosis… 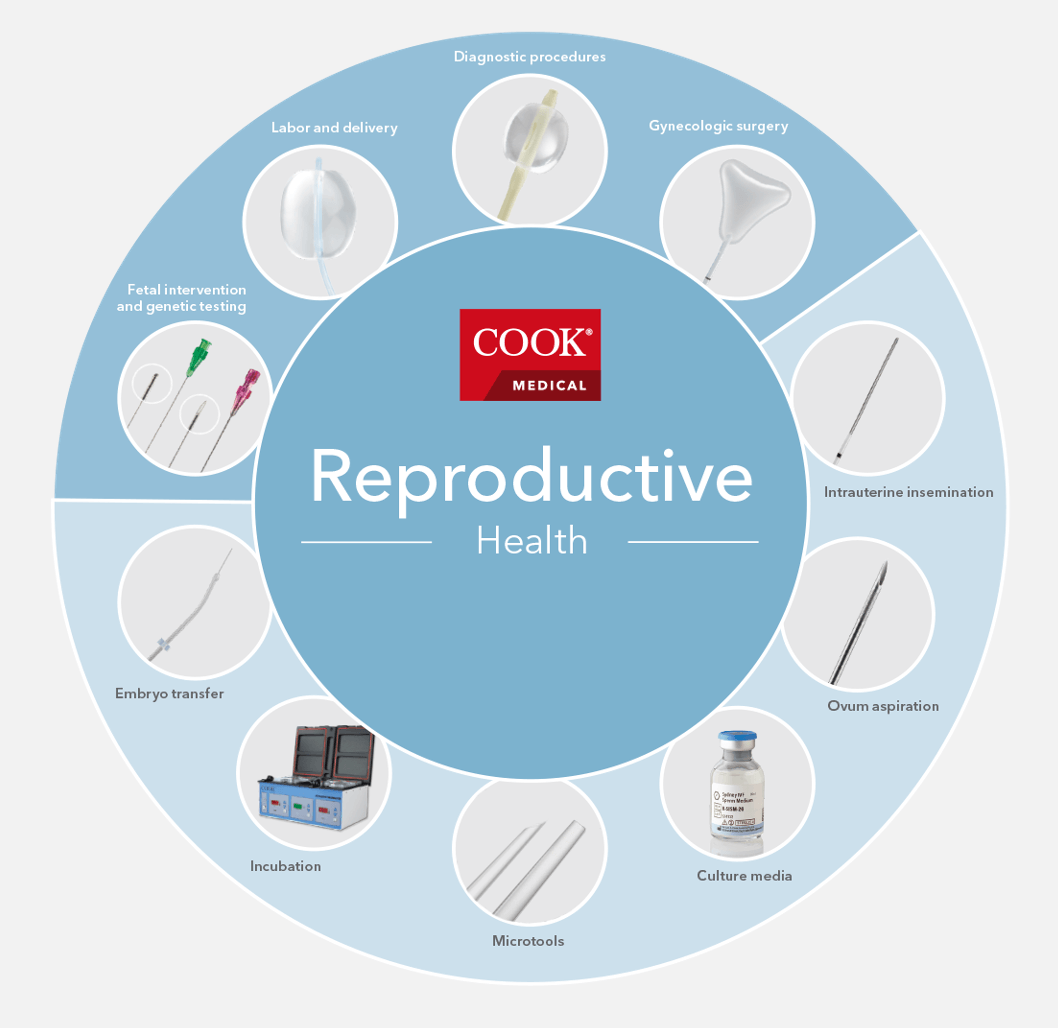 Bloomington, Indiana — Cook Medical today announced it signed a letter of intent with CooperCompanies to sell the entirety of Cook’s Reproductive Health business within the MedSurg division. CooperCompanies (NYSE:COO), a publicly held healthcare company, is focused on women’s health and fertility solutions, providing…
Bloomington, Indiana — Cook Medical today announced it signed a letter of intent with CooperCompanies to sell the entirety of Cook’s Reproductive Health business within the MedSurg division. CooperCompanies (NYSE:COO), a publicly held healthcare company, is focused on women’s health and fertility solutions, providing…  Indianapolis, Ind. – Indiana University’s Public Policy Institute (PPI) estimates that Bloomington, Indiana based Cook Medical’s medical device manufacturing facility nearing completion at 38th & Sheridan in Indianapolis will have an estimated economic impact of $25.9 million annually for Marion…
Indianapolis, Ind. – Indiana University’s Public Policy Institute (PPI) estimates that Bloomington, Indiana based Cook Medical’s medical device manufacturing facility nearing completion at 38th & Sheridan in Indianapolis will have an estimated economic impact of $25.9 million annually for Marion… 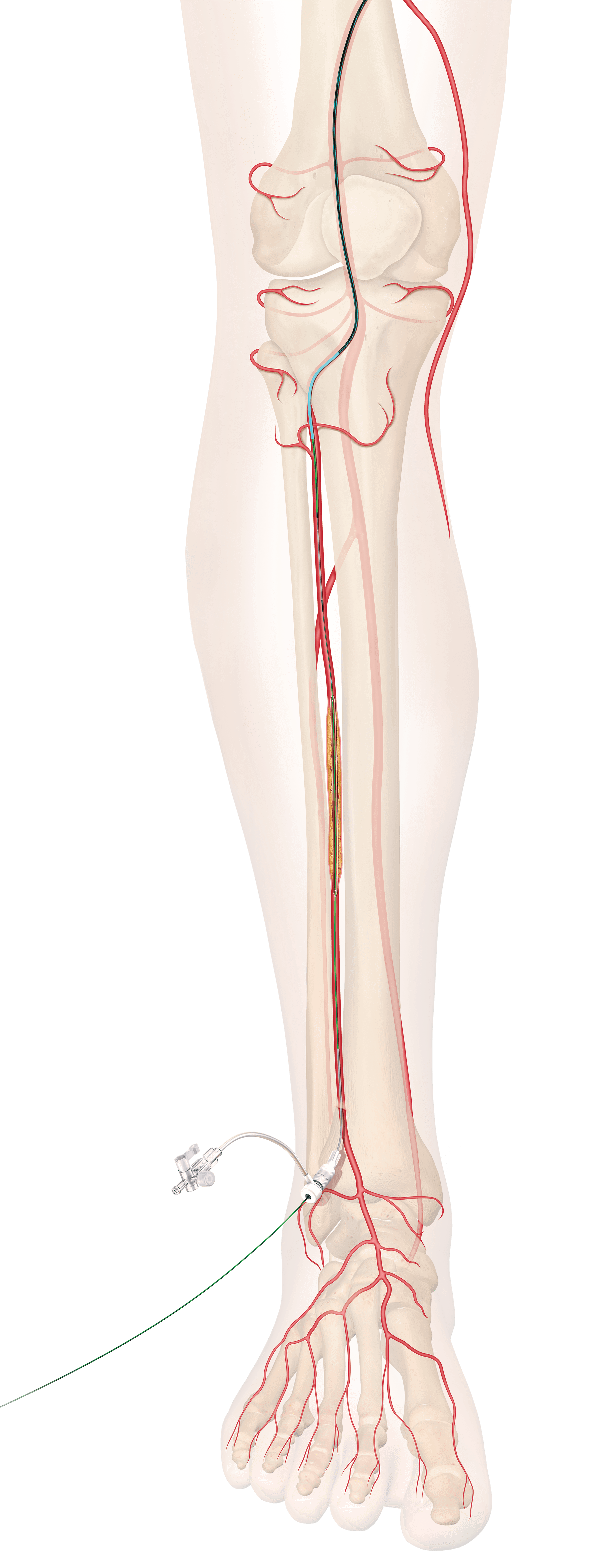 Bloomington, Ind. — Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee (BTK). This new stent is designed to treat patients suffering from chronic limb-threatening…
Bloomington, Ind. — Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee (BTK). This new stent is designed to treat patients suffering from chronic limb-threatening… 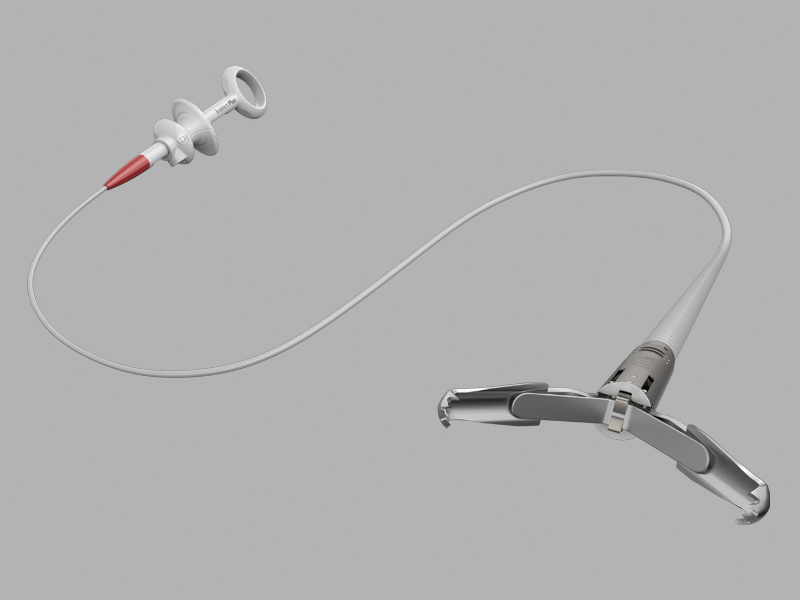 Bloomington, Ind. — Cook Medical’s Instinct Plus® Endoscopic Clipping Device is now commercially available to physicians in the United States. The new clipping device, based on the design of the original Instinct® Endoscopic Clip, features smoother operation and responsive handling1,…
Bloomington, Ind. — Cook Medical’s Instinct Plus® Endoscopic Clipping Device is now commercially available to physicians in the United States. The new clipping device, based on the design of the original Instinct® Endoscopic Clip, features smoother operation and responsive handling1,…  Bloomington, Ind. — Cook Medical has received a three-year contract from Vizient for peripheral vascular devices. The contract allows Cook Medical, which was recently named a finalist for the Vizient 2021 Physician Preference Supplier recognition, to offer negotiated pricing terms…
Bloomington, Ind. — Cook Medical has received a three-year contract from Vizient for peripheral vascular devices. The contract allows Cook Medical, which was recently named a finalist for the Vizient 2021 Physician Preference Supplier recognition, to offer negotiated pricing terms…  Indianapolis, Ind. — What started as a project to address a business challenge and expand manufacturing capacity for Cook Medical has turned into a community collaboration that includes a 100-job manufacturing site and a new grocery store called the Indy Fresh…
Indianapolis, Ind. — What started as a project to address a business challenge and expand manufacturing capacity for Cook Medical has turned into a community collaboration that includes a 100-job manufacturing site and a new grocery store called the Indy Fresh… 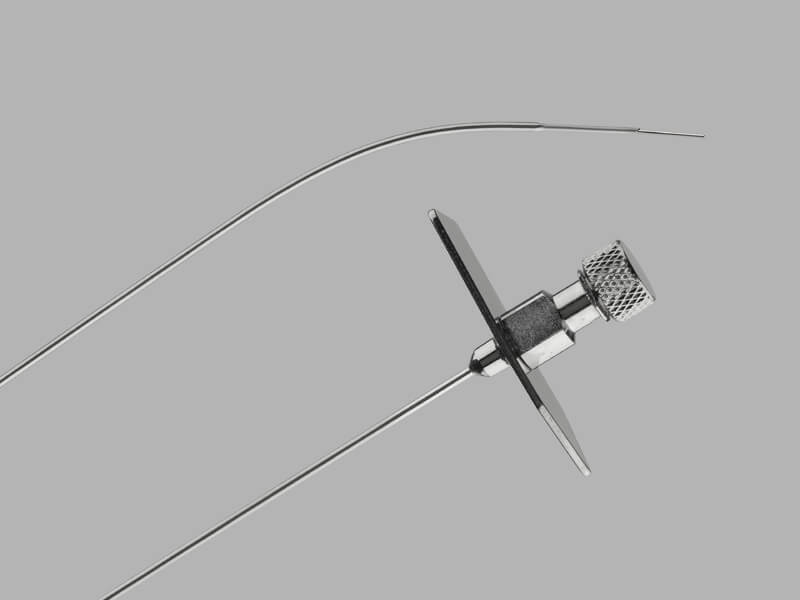 Bloomington, Ind. — On October 8, 2021, Cook Medical issued a global, voluntary recall of the Transseptal Needle and the Transseptal Needle with Catheter. This recall includes all unexpired lots for both of these products. The needles were recalled due…
Bloomington, Ind. — On October 8, 2021, Cook Medical issued a global, voluntary recall of the Transseptal Needle and the Transseptal Needle with Catheter. This recall includes all unexpired lots for both of these products. The needles were recalled due…  Canton, Ill. — Cook Medical is hiring over 100 manufacturing employees and five professional positions at our facility in Canton, Illinois. The expanded manufacturing capabilities will help Cook meet patient and customer needs for medical devices. In addition to providing…
Canton, Ill. — Cook Medical is hiring over 100 manufacturing employees and five professional positions at our facility in Canton, Illinois. The expanded manufacturing capabilities will help Cook meet patient and customer needs for medical devices. In addition to providing… 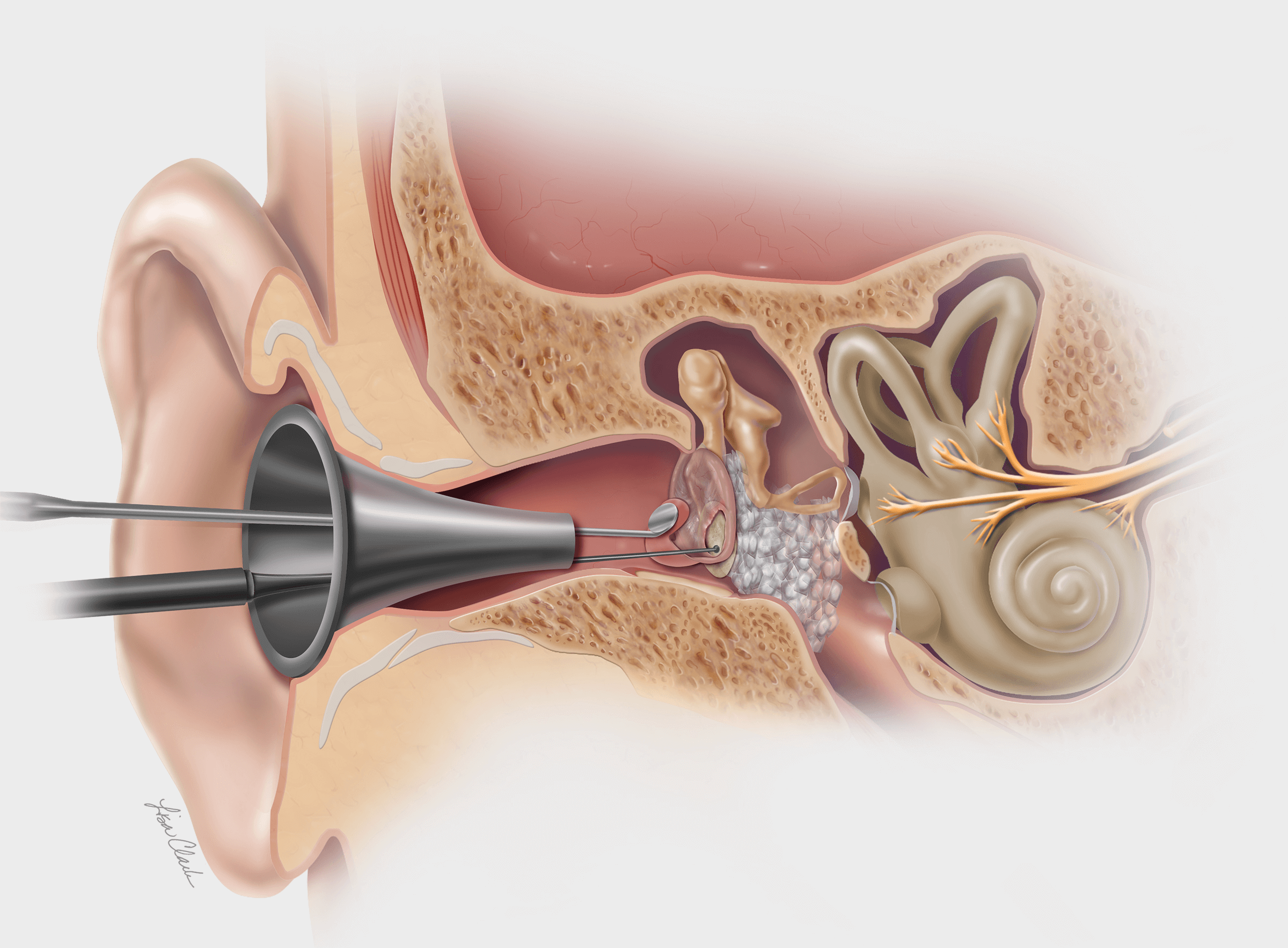 Bloomington, Ind. — Today, Cook Medical announced a new post-market study for the Biodesign Otologic Repair Graft. Otologic repair on perforated eardrums usually requires physicians to harvest patient tissue from elsewhere in the body to repair the membrane. This Cook…
Bloomington, Ind. — Today, Cook Medical announced a new post-market study for the Biodesign Otologic Repair Graft. Otologic repair on perforated eardrums usually requires physicians to harvest patient tissue from elsewhere in the body to repair the membrane. This Cook…  Bloomington, Ind. — A year without Cook Medical’s annual E-Recycle Day led to a record-breaking amount of e-waste collected to be recycled safely and sustainably. Not only was this a record for Cook’s annual Recycle Day, but the event was…
Bloomington, Ind. — A year without Cook Medical’s annual E-Recycle Day led to a record-breaking amount of e-waste collected to be recycled safely and sustainably. Not only was this a record for Cook’s annual Recycle Day, but the event was…  Farah Yousry, a health equity reporter with NPR’s WFYI, Side Effects News and the Indianapolis Recorder, recently shared a story on NPR’s All Things Considered about Cook Medical titled This Neighborhood Badly Needs A Grocery Store. A Medical Device Maker…
Farah Yousry, a health equity reporter with NPR’s WFYI, Side Effects News and the Indianapolis Recorder, recently shared a story on NPR’s All Things Considered about Cook Medical titled This Neighborhood Badly Needs A Grocery Store. A Medical Device Maker…  Demee Koch recently shared an interview with Cook Medical and Cook Group’s president in Medium’s post, Conscious Entrepreneurship: May I introduce Pete Yonkman. Our president, Pete Yonkman, believes companies need to develop deep connections with employees, customers and communities in…
Demee Koch recently shared an interview with Cook Medical and Cook Group’s president in Medium’s post, Conscious Entrepreneurship: May I introduce Pete Yonkman. Our president, Pete Yonkman, believes companies need to develop deep connections with employees, customers and communities in… 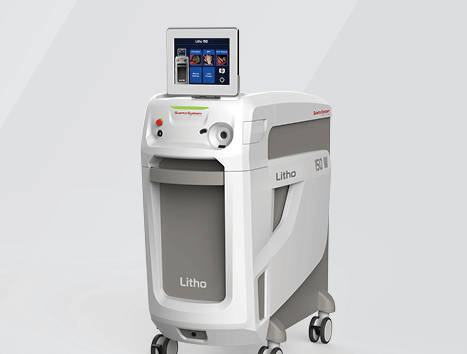 Bloomington, Ind. — The Litho 150 laser is now commercially available in the US. The product is the latest offering from Quanta System and is available through Cook Medical distribution. With power of up to 152 W, this ultra-high-power laser…
Bloomington, Ind. — The Litho 150 laser is now commercially available in the US. The product is the latest offering from Quanta System and is available through Cook Medical distribution. With power of up to 152 W, this ultra-high-power laser…  View news coverage of this announcement below. Indianapolis, Ind. — An Indianapolis neighborhood will soon have access to fresh food with a new grocery store, brought to life through a truly unique neighborhood, corporate and non-profit collaboration. The Indy Fresh…
View news coverage of this announcement below. Indianapolis, Ind. — An Indianapolis neighborhood will soon have access to fresh food with a new grocery store, brought to life through a truly unique neighborhood, corporate and non-profit collaboration. The Indy Fresh…  West Lafayette, Ind. — The FDA has reviewed the applications to the Accreditation Scheme for Conformity Assessment (ASCA) Pilot program and has granted laboratory safety accreditation to Cook Research Incorporated (CRI), a part of Cook Medical. CRI is the nonclinical…
West Lafayette, Ind. — The FDA has reviewed the applications to the Accreditation Scheme for Conformity Assessment (ASCA) Pilot program and has granted laboratory safety accreditation to Cook Research Incorporated (CRI), a part of Cook Medical. CRI is the nonclinical…  Cook Medical has a long history working with pioneers and innovators to develop products that are beneficial to both patients and clinicians. For Women’s History Month and beyond, we celebrate our current and past partnerships with women who made a difference. Product name: Rösch-Thurmond Fallopian Tube Catheterization Set…
Cook Medical has a long history working with pioneers and innovators to develop products that are beneficial to both patients and clinicians. For Women’s History Month and beyond, we celebrate our current and past partnerships with women who made a difference. Product name: Rösch-Thurmond Fallopian Tube Catheterization Set…  Bloomington, Ind. — Cook Medical today announced that the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays are commercially available to physicians in the United States and Canada. This product is a new iteration of the Ciaglia Blue Rhino…
Bloomington, Ind. — Cook Medical today announced that the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays are commercially available to physicians in the United States and Canada. This product is a new iteration of the Ciaglia Blue Rhino…  Bloomington, Ind. – The Litho EVO holmium laser is now available through Cook Medical in the United States, Austria, Germany, France, Ireland, Switzerland, and the United Kingdom. The Quanta Litho EVO laser is now available through…
Bloomington, Ind. – The Litho EVO holmium laser is now available through Cook Medical in the United States, Austria, Germany, France, Ireland, Switzerland, and the United Kingdom. The Quanta Litho EVO laser is now available through…  View news coverage of this announcement below. How a Unique Partnership Between Neighbors, a Medical Device Company, Government, and Non-Profits is Changing an Indianapolis Community Bloomington, Ind. – A unique partnership designed to bring jobs, free education, wrap around services…
View news coverage of this announcement below. How a Unique Partnership Between Neighbors, a Medical Device Company, Government, and Non-Profits is Changing an Indianapolis Community Bloomington, Ind. – A unique partnership designed to bring jobs, free education, wrap around services…  Bloomington, Ind. – Cook Medical announced today that the Hercules® 100 Transnasal Esophageal Balloon is now commercially available to physicians in the US. This product is the first balloon designed specifically for transnasal esophageal procedures. With the Hercules 100, ENT…
Bloomington, Ind. – Cook Medical announced today that the Hercules® 100 Transnasal Esophageal Balloon is now commercially available to physicians in the US. This product is the first balloon designed specifically for transnasal esophageal procedures. With the Hercules 100, ENT…  Bloomington, Ind., and Shaumburg, Il. – Dave Reed, retired vice president of Healthcare Business Solutions at Cook Medical, was recently inducted into the Hall of Fame for Healthcare Supply Chain Leadership by the Bellwether League, Inc. This year, the Bellwether…
Bloomington, Ind., and Shaumburg, Il. – Dave Reed, retired vice president of Healthcare Business Solutions at Cook Medical, was recently inducted into the Hall of Fame for Healthcare Supply Chain Leadership by the Bellwether League, Inc. This year, the Bellwether…  Bloomington, Ind. – A study recently published in BMC Urology compared Cook Medical’s Black Silicone Filiform Double Pigtail Ureteral Stent Set to a traditional polyurethane ureteral stent and found that on two separate patient surveys, the black silicone stent was…
Bloomington, Ind. – A study recently published in BMC Urology compared Cook Medical’s Black Silicone Filiform Double Pigtail Ureteral Stent Set to a traditional polyurethane ureteral stent and found that on two separate patient surveys, the black silicone stent was…  Bloomington, Ind. – Cook Medical today announced that the Advance Serenity™ Hydrophilic PTA Balloon Catheter is now available to physicians in the United States. The catheter is manufactured by Surmodics and distributed by Cook Medical. Advance Serenity is a hydrophilic-coated…
Bloomington, Ind. – Cook Medical today announced that the Advance Serenity™ Hydrophilic PTA Balloon Catheter is now available to physicians in the United States. The catheter is manufactured by Surmodics and distributed by Cook Medical. Advance Serenity is a hydrophilic-coated…  The Indianapolis Business Journal highlighted Cook Medical and Cook Group as one of the top 50 privately owned companies in Indiana. Cook Group ranked third in the state of Indiana this year. Reporter Sam Stall interviewed several Cook executives about…
The Indianapolis Business Journal highlighted Cook Medical and Cook Group as one of the top 50 privately owned companies in Indiana. Cook Group ranked third in the state of Indiana this year. Reporter Sam Stall interviewed several Cook executives about…  Bloomington, Ind. – Cook Medical is proud to announce the release of two products. The Transluminal Biliary Biopsy Forceps Set (BBFS) and a new 40-centimeter delivery system line extension of the existing Zilver 635® Biliary Stent (ZIB) are both now…
Bloomington, Ind. – Cook Medical is proud to announce the release of two products. The Transluminal Biliary Biopsy Forceps Set (BBFS) and a new 40-centimeter delivery system line extension of the existing Zilver 635® Biliary Stent (ZIB) are both now…  This is a painful time in our country. It’s unfathomable that tragedies such as the deaths of George Floyd, Breonna Taylor, and Ahmaud Arbery continue to happen. Sadly, as people of color know all too well, they do happen every…
This is a painful time in our country. It’s unfathomable that tragedies such as the deaths of George Floyd, Breonna Taylor, and Ahmaud Arbery continue to happen. Sadly, as people of color know all too well, they do happen every…  First, we want to thank everyone on the front lines fighting this pandemic. We acknowledge the sacrifices you are making to protect your own families while meeting your obligations to patients. Across the global healthcare industry, the COVID-19 pandemic has…
First, we want to thank everyone on the front lines fighting this pandemic. We acknowledge the sacrifices you are making to protect your own families while meeting your obligations to patients. Across the global healthcare industry, the COVID-19 pandemic has…  Cook Medical's TriForce® Peripheral Crossing Set Bloomington, Ind. – Cook Medical’s TriForce® Peripheral Crossing Set is now commercially available. As of January 2020, these products are available to physicians in the United States to support procedures to treat patients with…
Cook Medical's TriForce® Peripheral Crossing Set Bloomington, Ind. – Cook Medical’s TriForce® Peripheral Crossing Set is now commercially available. As of January 2020, these products are available to physicians in the United States to support procedures to treat patients with…  Bloomington, Ind. – The FDA has recently granted Cook Medical a De Novo authorization to market their new device, EchoTip® Insight™ Portosystemic Pressure Gradient Measurement System, in the United States. EchoTip Insight is an endoscopic ultrasound device which allows endoscopists…
Bloomington, Ind. – The FDA has recently granted Cook Medical a De Novo authorization to market their new device, EchoTip® Insight™ Portosystemic Pressure Gradient Measurement System, in the United States. EchoTip Insight is an endoscopic ultrasound device which allows endoscopists…  Bloomington, Ind. — Cook Medical’s innovative Hemospray® product received the 2019 Healio Disruptive Innovators Award in the Industry Breakthrough category. Healio, a healthcare news and education organization, presented the awards on October 27 at the American College of Gastroenterology Annual Scientific…
Bloomington, Ind. — Cook Medical’s innovative Hemospray® product received the 2019 Healio Disruptive Innovators Award in the Industry Breakthrough category. Healio, a healthcare news and education organization, presented the awards on October 27 at the American College of Gastroenterology Annual Scientific…  Hong Kong — Cook Medical announced today that Jean-Marc Creissel has been named vice president and director of Asia-Pacific. In his new role, Creissel will lead the Asia-Pacific teams to transform Cook Medical into…
Hong Kong — Cook Medical announced today that Jean-Marc Creissel has been named vice president and director of Asia-Pacific. In his new role, Creissel will lead the Asia-Pacific teams to transform Cook Medical into…  Phoenix, Ariz. — Cook Medical is proud to announce that Dave Reed, vice president of Healthcare Solutions, received the Chuck Lauer Award at the Integrated Delivery Network (IDN) Summit on September 10. Dave Reed (left) receives…
Phoenix, Ariz. — Cook Medical is proud to announce that Dave Reed, vice president of Healthcare Solutions, received the Chuck Lauer Award at the Integrated Delivery Network (IDN) Summit on September 10. Dave Reed (left) receives…  Bloomington, Ind. — Forbes named Cook Medical one of America’s Best Employers for Women 2019. We are honored to receive this award in addition to the Forbes rankings as one of America’s Best Employers for both 2018 and 2019. To rank…
Bloomington, Ind. — Forbes named Cook Medical one of America’s Best Employers for Women 2019. We are honored to receive this award in addition to the Forbes rankings as one of America’s Best Employers for both 2018 and 2019. To rank…  Abu Dhabi, UAE — Shayna Martin, global brand marketing director for Cook Medical’s Urology specialty, will receive the Endourological Society 2019 Industry Award during the society’s…
Abu Dhabi, UAE — Shayna Martin, global brand marketing director for Cook Medical’s Urology specialty, will receive the Endourological Society 2019 Industry Award during the society’s…  Bloomington, Ind. — Cook is pleased to announce that Rose Kelly-Falls has joined Cook Medical as global leader of procurement and supply chain. Kelly-Falls and…
Bloomington, Ind. — Cook is pleased to announce that Rose Kelly-Falls has joined Cook Medical as global leader of procurement and supply chain. Kelly-Falls and…  Bloomington, Ind. – A new animal study concludes that fewer fibered embolization coils are needed to achieve acute occlusion when compared with similar bare metal coils. The study was presented today at an industry sponsored symposium during the 2019 annual…
Bloomington, Ind. – A new animal study concludes that fewer fibered embolization coils are needed to achieve acute occlusion when compared with similar bare metal coils. The study was presented today at an industry sponsored symposium during the 2019 annual…  Bloomington, Ind. — Last week, Cook Medical was recognized as one of the best places to work in Bloomington. The honor comes from the Bloomington Economic Development Corporation in partnership with…
Bloomington, Ind. — Last week, Cook Medical was recognized as one of the best places to work in Bloomington. The honor comes from the Bloomington Economic Development Corporation in partnership with…  Bloomington, Ind. – Last week, Dave Reed was recognized as a leader in healthcare operations by the Global Healthcare Exchange (GHX). The Supply Chain Leadership award recognizes companies and individuals who make a difference in healthcare, transforming supply chain automation,…
Bloomington, Ind. – Last week, Dave Reed was recognized as a leader in healthcare operations by the Global Healthcare Exchange (GHX). The Supply Chain Leadership award recognizes companies and individuals who make a difference in healthcare, transforming supply chain automation,…  Bloomington, Ind. – The FDA has granted Cook Medical approval to market Hemospray®, in the United States. An endoscopic hemostat, Hemospray achieves hemostasis with a proprietary inorganic powder. Bleeding in the gastrointestinal tract is a…
Bloomington, Ind. – The FDA has granted Cook Medical approval to market Hemospray®, in the United States. An endoscopic hemostat, Hemospray achieves hemostasis with a proprietary inorganic powder. Bleeding in the gastrointestinal tract is a…  Bloomington, Ind. – Cook Medical has announced Ross Harvey as the new director of global Customer Support & Delivery (CSD). In this role, Harvey will oversee the global customer service teams, global distribution centers, and warehouses. These teams will work…
Bloomington, Ind. – Cook Medical has announced Ross Harvey as the new director of global Customer Support & Delivery (CSD). In this role, Harvey will oversee the global customer service teams, global distribution centers, and warehouses. These teams will work…  Bloomington, Ind. – Today, Christina Anné was named vice president of Distribution Channel Management (DCM) for Cook Medical. The DCM team will be dedicated to serving the needs of Cook’s distributors around the world and to ensure compliant and ethical…
Bloomington, Ind. – Today, Christina Anné was named vice president of Distribution Channel Management (DCM) for Cook Medical. The DCM team will be dedicated to serving the needs of Cook’s distributors around the world and to ensure compliant and ethical…  Bloomington, Ind. – District Court Judge Richard L. Young entered judgment in favor of Cook Medical in the second bellwether case on IVC filters.…
Bloomington, Ind. – District Court Judge Richard L. Young entered judgment in favor of Cook Medical in the second bellwether case on IVC filters.…  Bloomington, Ind. – Today, Cook Medical announced Dan Kaiser as the leader of global research and development (R&D). This dedicated engineering team will connect product development more closely to customer needs and accelerate the process of getting new products to…
Bloomington, Ind. – Today, Cook Medical announced Dan Kaiser as the leader of global research and development (R&D). This dedicated engineering team will connect product development more closely to customer needs and accelerate the process of getting new products to…  Bloomington, Ind. – Today, Cook Medical announced key changes that simplify its organizational structure to better support customers. These changes realign the current sales, marketing, research and development, and customer service teams in addition to establishing new distribution channel management…
Bloomington, Ind. – Today, Cook Medical announced key changes that simplify its organizational structure to better support customers. These changes realign the current sales, marketing, research and development, and customer service teams in addition to establishing new distribution channel management…  Bloomington, Ind. – Jean-Marc Creissel received the Endourology Society 2017 Industry Award during the society’s annual World Congress of Endourology and SWL in Vancouver. Creissel is vice president of Cook Medical’s…
Bloomington, Ind. – Jean-Marc Creissel received the Endourology Society 2017 Industry Award during the society’s annual World Congress of Endourology and SWL in Vancouver. Creissel is vice president of Cook Medical’s…  Bloomington, Ind. – April marked the first anniversary of Cook’s tutoring program in partnership with Bloomington’s Crestmont Boys & Girls Club. Over the past year, 30…
Bloomington, Ind. – April marked the first anniversary of Cook’s tutoring program in partnership with Bloomington’s Crestmont Boys & Girls Club. Over the past year, 30…  West Lafayette, Ind. – Jennifer Kerr, president of Cook Research, was named to the 2017 class of Hoosier Fellows through the Randall L. Tobias Center for Leadership Excellence at Indiana University. Participants in the Hoosier Fellows program come from across…
West Lafayette, Ind. – Jennifer Kerr, president of Cook Research, was named to the 2017 class of Hoosier Fellows through the Randall L. Tobias Center for Leadership Excellence at Indiana University. Participants in the Hoosier Fellows program come from across…  Bloomington, Ind. – Students from Ivy Tech Community College Bloomington have collaborated with Cook Medical and Zumasys through real-life computer programming coursework on jBASE, a database management system used by manufacturers worldwide. Zumasys, a cloud computing platform and owner of…
Bloomington, Ind. – Students from Ivy Tech Community College Bloomington have collaborated with Cook Medical and Zumasys through real-life computer programming coursework on jBASE, a database management system used by manufacturers worldwide. Zumasys, a cloud computing platform and owner of…  Bloomington, Ind.— Cook Medical has rounded out the Universa line with the introduction of two new sets for percutaneous urinary drainage. Each set includes a catheter and accessories for specific procedures. These…
Bloomington, Ind.— Cook Medical has rounded out the Universa line with the introduction of two new sets for percutaneous urinary drainage. Each set includes a catheter and accessories for specific procedures. These…  Bloomington, Ind.— Cook Medical announced the introduction of a two-in-one wire guide, the Motion™ Hybrid Wire Guide. This wire guide combines the features of a nitinol access wire guide and a Teflon™…
Bloomington, Ind.— Cook Medical announced the introduction of a two-in-one wire guide, the Motion™ Hybrid Wire Guide. This wire guide combines the features of a nitinol access wire guide and a Teflon™…  Bloomington, Ind. – Cook Medical is pleased to announce that Jean-Marc Creissel has been named vice president of operations for the greater China region. In his new role, Creissel will work with the global and regional teams in APAC to…
Bloomington, Ind. – Cook Medical is pleased to announce that Jean-Marc Creissel has been named vice president of operations for the greater China region. In his new role, Creissel will work with the global and regional teams in APAC to…  Cook Group and Cook Medical sat down with The Herald-Times, the local newspaper in Bloomington, Indiana, to discuss Cook's transformation and preparation for healthcare of the future. These interviews resulted in a four-day series by veteran reporter Bill Strother as described…
Cook Group and Cook Medical sat down with The Herald-Times, the local newspaper in Bloomington, Indiana, to discuss Cook's transformation and preparation for healthcare of the future. These interviews resulted in a four-day series by veteran reporter Bill Strother as described…  Bloomington, Ind. – Pete Yonkman, Cook Medical and Cook Group president, appeared on Inside INdiana Business with Gerry Dick on July 10, 2016, to discuss Cook's new partnership with Broadview Learning Center and Ivy Tech Community College to create a…
Bloomington, Ind. – Pete Yonkman, Cook Medical and Cook Group president, appeared on Inside INdiana Business with Gerry Dick on July 10, 2016, to discuss Cook's new partnership with Broadview Learning Center and Ivy Tech Community College to create a…  Bloomington, Ind. – Today, Cook Group, Monroe County Community Schools Adult Education at Broadview Learning Center and Ivy Tech Community College Bloomington announced their partnership to create a new pathway between education and jobs in our community. According to the…
Bloomington, Ind. – Today, Cook Group, Monroe County Community Schools Adult Education at Broadview Learning Center and Ivy Tech Community College Bloomington announced their partnership to create a new pathway between education and jobs in our community. According to the…  Limerick, Ireland – In 2016 Cook Medical will celebrate 20 years of operations in the National Technology Park where it has grown from a primary team of less than a dozen people to a staff of over 800, who are…
Limerick, Ireland – In 2016 Cook Medical will celebrate 20 years of operations in the National Technology Park where it has grown from a primary team of less than a dozen people to a staff of over 800, who are…  Bloomington, Ind. – Cook Group is pleased to announce the promotion of Barry Slowey to president of Cook Endoscopy/Cook Winston-Salem. As president, Slowey will oversee the various functions in Winston-Salem, North Carolina, and will maintain his leadership responsibilities for the…
Bloomington, Ind. – Cook Group is pleased to announce the promotion of Barry Slowey to president of Cook Endoscopy/Cook Winston-Salem. As president, Slowey will oversee the various functions in Winston-Salem, North Carolina, and will maintain his leadership responsibilities for the…  Bloomington, Ind. – Cook Medical’s Endoscopy clinical division announces the introduction of the latest addition to the EchoTip ProCore® product line, the EchoTip ProCore 20 gage needle with ReCoil Stylet™. This needle’s flexible design allows physicians to obtain histological samples…
Bloomington, Ind. – Cook Medical’s Endoscopy clinical division announces the introduction of the latest addition to the EchoTip ProCore® product line, the EchoTip ProCore 20 gage needle with ReCoil Stylet™. This needle’s flexible design allows physicians to obtain histological samples…  Bloomington, Ind. – Cook Medical has been recognized as a Gold-Level Fit-Friendly Worksite by the American Heart Association for helping employees eat better…
Bloomington, Ind. – Cook Medical has been recognized as a Gold-Level Fit-Friendly Worksite by the American Heart Association for helping employees eat better…  Bloomington, Ind. — Cook Group announced today that Kem Hawkins, president of Cook Group Incorporated, will retire on July 1 after 34 years with the Cook organization. Hawkins, who has been transitioning his role in the company over the past…
Bloomington, Ind. — Cook Group announced today that Kem Hawkins, president of Cook Group Incorporated, will retire on July 1 after 34 years with the Cook organization. Hawkins, who has been transitioning his role in the company over the past…  Bloomington, Ind., January 26, 2015 – Cynthia Kretz, who has served as general counsel since 2008, has been promoted to vice president general counsel, the company said today. As vice president, Kretz will continue her responsibility leading the global operations…
Bloomington, Ind., January 26, 2015 – Cynthia Kretz, who has served as general counsel since 2008, has been promoted to vice president general counsel, the company said today. As vice president, Kretz will continue her responsibility leading the global operations…  Las Vegas, Nev., November 4, 2014 — Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) confirmed long-term patency for patients treated with Zilver PTX. The results were presented today by…
Las Vegas, Nev., November 4, 2014 — Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) confirmed long-term patency for patients treated with Zilver PTX. The results were presented today by…  Bloomington, Ind., September 24, 2014 – Connie Jackson, who has served as global vice president of human resources since 2005, has been promoted to senior vice president of human resources, the company said today. As senior vice president, Jackson will continue…
Bloomington, Ind., September 24, 2014 – Connie Jackson, who has served as global vice president of human resources since 2005, has been promoted to senior vice president of human resources, the company said today. As senior vice president, Jackson will continue…  Vandergrift, Pa. — Effective immediately, Cook Medical customers in the United States will again have access to its Evolution® RL and Evolution® Shortie RL Controlled-Rotation Dilator Sheath Sets. Distribution of these products within the U.S. market was suspended for two months while…
Vandergrift, Pa. — Effective immediately, Cook Medical customers in the United States will again have access to its Evolution® RL and Evolution® Shortie RL Controlled-Rotation Dilator Sheath Sets. Distribution of these products within the U.S. market was suspended for two months while…  Bloomington, Ind., April 21, 2014 – The Instinct® Endoscopic Clip is now available to gastroenterologists in major global markets. The clip is used to stop gastrointestinal (GI) tract bleeding, which is a condition that can be challenging to treat because of…
Bloomington, Ind., April 21, 2014 – The Instinct® Endoscopic Clip is now available to gastroenterologists in major global markets. The clip is used to stop gastrointestinal (GI) tract bleeding, which is a condition that can be challenging to treat because of…  Bloomington, Ind. – In response to the NAP4 report addressing the clinical need for both Seldinger* and surgical cricothyrotomy procedures to be taught side-by-side, Cook Medical today announces a compact surgical set. This cricothyrotomy range expansion allows clinicians to stay up-to-date…
Bloomington, Ind. – In response to the NAP4 report addressing the clinical need for both Seldinger* and surgical cricothyrotomy procedures to be taught side-by-side, Cook Medical today announces a compact surgical set. This cricothyrotomy range expansion allows clinicians to stay up-to-date…  Bloomington, Ind. – CFC Properties, a Cook Group company, welcomes a new vice president of commercial real estate. Ron Walker, former president of the Bloomington Economic Development Corporation (BEDC), is looking forward to his new position. "I am truly…
Bloomington, Ind. – CFC Properties, a Cook Group company, welcomes a new vice president of commercial real estate. Ron Walker, former president of the Bloomington Economic Development Corporation (BEDC), is looking forward to his new position. "I am truly…  Bloomington, Ind. – Cook Medical has launched a new treatment option for otolaryngologists (ear, nose and throat specialists) who repair the dura mater following cerebrospinal fluid (CSF) leaks at the base of the skull. Cook’s Biodesign® Duraplasty Graft is the…
Bloomington, Ind. – Cook Medical has launched a new treatment option for otolaryngologists (ear, nose and throat specialists) who repair the dura mater following cerebrospinal fluid (CSF) leaks at the base of the skull. Cook’s Biodesign® Duraplasty Graft is the…  Bloomington, Ind.—Cook Medical is introducing the first endobronchial ultrasound (EBUS) needle in the U.S. and Europe that can acquire histological samples. The EchoTip® ProCore™ Endobronchial Ultrasound Needle gives physicians the ability to retrieve both cell and tissue samples from lymph…
Bloomington, Ind.—Cook Medical is introducing the first endobronchial ultrasound (EBUS) needle in the U.S. and Europe that can acquire histological samples. The EchoTip® ProCore™ Endobronchial Ultrasound Needle gives physicians the ability to retrieve both cell and tissue samples from lymph…  Winston-Salem, N.C.—Cook Medical has initiated a clinical study in the U.S. to evaluate the removability of a new Evolution® Esophageal Fully Covered Stent. This is the first multicenter U.S. study to evaluate the possibility of removing a self-expanding metal stent…
Winston-Salem, N.C.—Cook Medical has initiated a clinical study in the U.S. to evaluate the removability of a new Evolution® Esophageal Fully Covered Stent. This is the first multicenter U.S. study to evaluate the possibility of removing a self-expanding metal stent…  Bloomington, Ind. – Mark Breedlove, a 19-year veteran of Cook Medical, has been named global leader for the company’s Peripheral Interventional (PI) division. “As our largest division, PI plays a vital role in Cook Medical’s global success,” said Pete Yonkman, president…
Bloomington, Ind. – Mark Breedlove, a 19-year veteran of Cook Medical, has been named global leader for the company’s Peripheral Interventional (PI) division. “As our largest division, PI plays a vital role in Cook Medical’s global success,” said Pete Yonkman, president…  Bloomington, Ind. – Rob Lyles, a veteran leader of Cook Medical’s Peripheral Intervention (PI) division, has been promoted to executive vice president in a move to further invigorate Cook’s drive toward new, cutting edge technologies and patient therapies. He will transition…
Bloomington, Ind. – Rob Lyles, a veteran leader of Cook Medical’s Peripheral Intervention (PI) division, has been promoted to executive vice president in a move to further invigorate Cook’s drive toward new, cutting edge technologies and patient therapies. He will transition…  Bloomington, Ind. – Kem Hawkins, president of Cook Group Incorporated, announced today that Pete Yonkman will lead Cook Group’s medical companies as president of Cook Medical. Hawkins will continue serving as president of Cook Group. “Cook is an amazing company run…
Bloomington, Ind. – Kem Hawkins, president of Cook Group Incorporated, announced today that Pete Yonkman will lead Cook Group’s medical companies as president of Cook Medical. Hawkins will continue serving as president of Cook Group. “Cook is an amazing company run…  Bloomington, Ind. — Cook Medical has today officially opened its new state-of-the-art research and development (R&D) Innovation Centre at its plant in Limerick, Ireland. The Innovation Centre together, with the expansion of cleanrooms, packaging, storage and other facilities, represents…
Bloomington, Ind. — Cook Medical has today officially opened its new state-of-the-art research and development (R&D) Innovation Centre at its plant in Limerick, Ireland. The Innovation Centre together, with the expansion of cleanrooms, packaging, storage and other facilities, represents…  New Orleans, La. – The 2013 Arthur D. Smith Endourology Lectureship has been awarded to Thomas Knoll, MD, Associate Professor of Urology at University Medical Center Mannheim and the head of the Department of Urology at the Sindelfingen Medical center of…
New Orleans, La. – The 2013 Arthur D. Smith Endourology Lectureship has been awarded to Thomas Knoll, MD, Associate Professor of Urology at University Medical Center Mannheim and the head of the Department of Urology at the Sindelfingen Medical center of…  Bloomington, Ind. – Gretchen Gutman, a 20-year veteran of legislative and government policy, has joined Cook Group as vice president of public policy. “The health care industry is constantly facing changes and issues at all levels of government policy,” said Dan…
Bloomington, Ind. – Gretchen Gutman, a 20-year veteran of legislative and government policy, has joined Cook Group as vice president of public policy. “The health care industry is constantly facing changes and issues at all levels of government policy,” said Dan…