CAUTION – INVESTIGATIONAL DEVICE. Limited by United States law to investigational use.
Bloomington, Ind. — Cook Medical today announced the first patient treated in the clinical study of the ZENITH® FENESTRATED+ Endovascular Graft (ZFEN+) in the United States. The procedure was performed on January 29 at UT Health Houston in Houston, Texas, by Gustavo Oderich, MD, Global Principal Investigator. 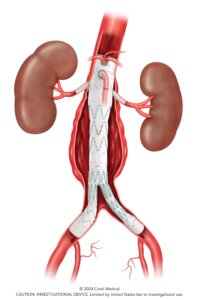
“We are ecstatic at UT Health Houston to have treated this first ZFEN+ patient. This is a huge step forward in getting the first patient-specific device for complex abdominal and extent IV thoracoabdominal aneurysms with fenestrations. Rather than a one-size-fits-all approach, the ZFEN+ device design aims to optimize patient needs,” said Dr. Oderich.
The ZFEN+ clinical study is being conducted under an Investigational Device Exemption (IDE) from the U.S. Food and Drug Administration (FDA). This clinical study will assess the safety and effectiveness of the ZFEN+ used in combination with the investigational Zenith® Universal Distal Body 2.0 Graft (Unibody2), the investigational Bentley BeGraft Balloon-Expandable FEVAR Bridging Stent Graft System and the commercially available Zenith® Spiral-Z® AAA Iliac Leg Graft (ZSLE).
The ZFEN+ clinical study will enroll patients in need of treatment of aortic aneurysms involving one or more of the major visceral arteries. Patients with Juxtarenal, Pararenal and Extent IV thoracoabdominal aneurysms meeting specific anatomical requirements may be included in the study. The ZFEN+ device is designed with fenestrations aligned to a patient’s unique anatomy and to extend the proximal margin of aneurysmal disease that can be treated endovascularly.
“On behalf of Cook Medical, we are very excited to announce the first implant of ZFEN+. This is an exciting milestone in the advancement of fenestrated aortic technology. This next generation of patient-specific aortic endograft technology has received a Breakthrough Device Designation from the FDA. This advancement will enable physicians to select up to five target vessels. Our team is committed to listening, collaborating and bringing these innovative technologies to a broader patient population,” said Johnny LeBlanc, director of aortic product management at Cook Medical.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
News coverage of this announcement
Endovascular Today–Cook Medical Enrolls First Patient in ZFEN+ Clinical Study
Vascular News–Cook Medical reports first patient treated in ZFEN+ fenestrated endovascular graft study