August 20th, 2025

CAUTION: Investigational Device. Limited by United States law to investigational use. Bloomington, Ind. — The U.S. Food and Drug Administration (FDA) has granted approval…
August 17th, 2025
Bloomington, Ind. — Gayle Karch Cook, historical preservation activist and cofounder of the Cook companies alongside husband Bill Cook, passed away at 91 this morning. She is survived by her son Carl and daughter-in-law Marcy and their granddaughter, Eleanor. “Gayle’s spirit of…
August 15th, 2025

We are passionate about innovation in the supply chain industry. Our goal is to connect with and listen to healthcare professionals to identify unmet needs and use our expertise to imagine new solutions. For years, we have consistently heard that…
July 28th, 2025

Leveraging Cook's history of innovative medical devices in a new procedural setting Bloomington, Ind. — Cook Medical today announced the launch of its new Interventional Magnetic Resonance Imaging (iMRI) division, marking a significant step forward in its commitment to transforming…
July 17th, 2025

Cook Medical has released its Social Impact & Sustainability Report for 2024, highlighting the company’s commitment to working sustainably and responsibly while fulfilling its purpose to improve the lives of everyone it serves. The report reflects on the achievements of Cook's teams across…
July 14th, 2025
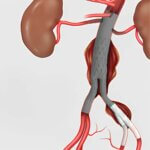
Bloomington, Ind. — Cook Medical’s Zenith® Iliac Branch Device (ZBIS) is now commercially available in the United States with FDA approval as an endovascular treatment option for aortoiliac or…
April 23rd, 2025

Bloomington, Ind. — Cook Medical announced a collaboration with Mendaera™ Inc., a Silicon Valley-based healthcare technology company. The goal of this collaboration is to create cutting-edge solutions for needle-based interventions in urological procedures by combining Cook's market-leading needles with Mendaera's handheld…
April 3rd, 2025
Please join us as we highlight some of our leaders, learn about their experiences, and hear how they are talking about the positive changes we’re making to meet our vision of the future at Cook. Follow along for the full Meet…
March 27th, 2025
Bloomington, Ind. — Cook Medical’s Zilver PTX drug-eluting stent (DES) has lower rates of in-stent occlusions among patients with restenosis at three years than Boston Scientific’s Eluvia DES, according to real-world data from the REALDES study. The data, published by…
March 10th, 2025

Our mission stays the same, but at Cook, we’re relentlessly inventive and proactively adapting to the needs of physicians and patients. We previously shared three ways Cook is changing. Since then, we’ve made other significant advancements. Here are four more…
March 7th, 2025
Please join us as we highlight some of our leaders, learn about their experiences, and hear how they are talking about the positive changes we’re making to meet our vision of the future at Cook. Follow along for the full Meet…
March 7th, 2025

Bloomington, Ind. — Cook Medical is now offering Magneto technology on the Quanta System 100W and 150W High Power Ho:YAG Laser Systems. Magneto technology allows physicians to perform a wider array of endourological procedures than ever before with one laser…
March 6th, 2025

Bloomington, Ind. — Cook Medical’s Zenith Alpha® 2 Thoracic Endovascular Graft (ZTA2) is now commercially available throughout the United States. ZTA2 is the first aortic device on the market that has evolved to accommodate CO2 flushing and has an approved…
January 31st, 2025
Please join us as we highlight some of our leaders, learn about their experiences, and hear how they are talking about the positive changes we’re making to meet our vision of the future at Cook. Follow along for the full Meet…
November 22nd, 2024

Please join us as we highlight some of our leaders, learn about their experiences, and hear how they are talking about the positive changes we’re making to meet our vision of the future at Cook. Follow along for the full Meet…
November 18th, 2024

Bloomington, Ind. — Furthering their commitment to relentlessly delivering new products and services to address unmet customer needs, today Cook Medical announced a strategic investment in the urology space. Zenflow is a medical device company developing a minimally invasive treatment…
October 29th, 2024
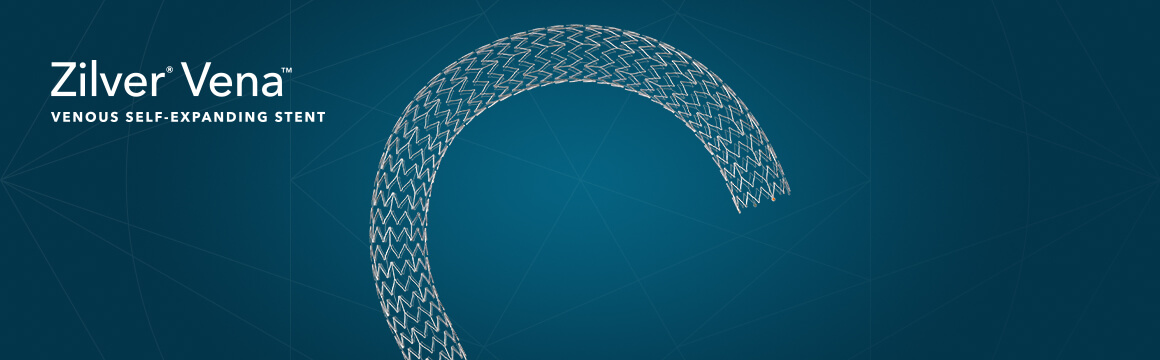
Bloomington, Ind. — Cook Medical’s Zilver Vena® Venous Self-Expanding Stent has shown high rates of patency sustained for three years, according to recently published data in the Journal of Vascular and Interventional Radiology (JVIR).1 The patency results extended across all patient…
October 22nd, 2024

Bloomington, Ind. — Cook Medical is now distributing the PillSense™ GI Bleed Detection System in the United States. PillSense™ is a novel device that can detect upper gastrointestinal (GI) bleeding in patients in under 10 minutes. Cook is constantly changing…
October 17th, 2024

Bloomington, Ind. — Cook Medical is now offering the NestVT Vitrification Device in the United States. This device is a secure solution for embryos and oocytes during vitrification, cryo-storage and relocation. Along with the launch of this innovative product, Cook…
September 23rd, 2024
Please join us as we highlight some of our leaders, learn about their experiences, and hear how they are talking about the positive changes we’re making to meet our vision of the future at Cook. Follow along for the full Meet…
September 17th, 2024

Bloomington, Ind. — Cook Medical announced this morning it has signed an agreement with Merit Medical to purchase Cook’s Lead Management portfolio. This announcement is the conclusion of a series of planned Cook divestures across multiple product lines and specialties…
July 22nd, 2024

Bloomington, Ind. — Cook Medical announced this morning it has signed a letter of intent with Astorg, a leading private equity firm with an extensive and successful track record in global healthcare investments, to purchase Cook’s Reproductive Health business (“Cook…
June 27th, 2024

Bloomington, Ind. — Ross Whittaker, a manager of manufacturing engineering at Cook, was named a member of the Conexus Rising 30 Class of 2024 for his exceptional work in advanced manufacturing and logistics (AML) and the changes he and his…
June 26th, 2024

At Cook, our goal has always been to provide devices and services to support minimally invasive procedures for patients. That hasn’t changed. However, what has changed is the way we work toward that goal. In November 2023, we announced an…
January 8th, 2024
Bloomington, Ind. — Cook Medical’s Slip-Cath® Beacon® Tip Hydrophilic Selective Catheter is now available for use in the U.S. and Canada. Slip-Cath…
December 18th, 2023

Bloomington, Ind. — Cook Medical won the 2023 Loyal Partner Supplier Award from Capstone Health Alliance for outstanding work from Cook’s MedSurg division. Cook is proud…
November 7th, 2023

The last several years have brought significant change to Cook. To ensure that we are positioned to be successful in this new world, our global leadership teams have implemented a new vision and strategic plan that were informed by extensive…
November 1st, 2023
Bloomington, Indiana — In alignment with its 5-year strategic plan, Cook Medical announced today that CooperCompanies has acquired select products from Cook’s Maternal Fetal Medicine portfolio, as well as gynecological surgery products, and Doppler monitor technology. CooperCompanies (Nasdaq: COO), a…
October 18th, 2023
Bloomington, Ind. — The US Department of Veterans Affairs (VA) has awarded Cook Medical a Next Generation Implant Contract for implanted medical devices. This contract will streamline the procurement process for the VA supply chain team. This contract is another…
September 29th, 2023

Bloomington, Ind. — Cook Medical is pleased to promote Barry Slowey to vice president and chief sustainability officer. His new position was effective September 8, 2023. In his new appointment, Barry will provide leadership and implement the goals of Cook’s Social…
September 26th, 2023

Bloomington, Ind. — At the 2023 Vizient Connections Summit, Cook Medical won the Vizient Supply Assurance Supplier of the Year Award. This award demonstrates Cook’s transparency and commitment to communication during the supply chain process with healthcare organizations that are…
September 1st, 2023

Bloomington, Ind. — Cook Medical is pleased to promote Amber Beauchamp to vice president, global human resources at Cook Group and Cook Medical effective September 1, 2023. Beauchamp has more than 17 years of HR experience in multiple industries. She…
August 7th, 2023
Gina W. shares her diagnoses story and advocates for disease awareness In an open, honest, and, at times, emotional conversation, Gina W., a national sales manager for Cook, sat down with interviewer Kelly R., a Clinical Training manager for Cook,…
July 25th, 2023

Bringing the latest in medical technologies to the youngest patients INDIANAPOLIS and WEST LAFAYETTE, Ind. – Purdue University, the Indiana University School of Medicine and medical device company Cook Medical are focusing a new alliance on clinical needs in pediatrics…
July 17th, 2023

Cook applauds the U.S. Food and Drug Administration’s recent update on paclitaxel-coated devices. We believe this is the best decision for physicians and patients. “We are grateful for the FDA’s latest update on paclitaxel. We applaud decisions that are based…
June 29th, 2023
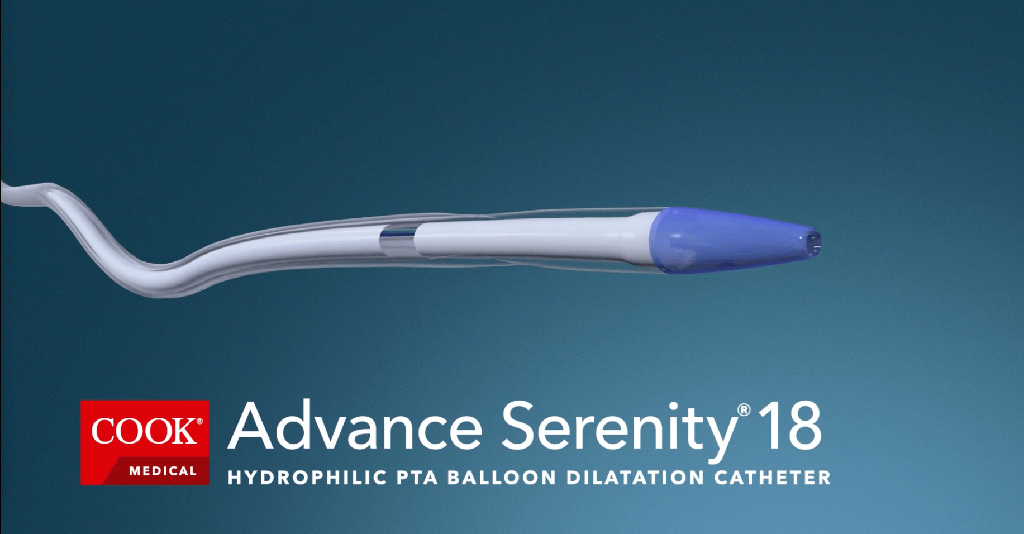
Bloomington, Ind. — As announced at the 2023 Vascular Annual Meeting (VAM), the Advance Serenity® Hydrophilic PTA Balloon Catheter product line is now available with even more options. Interventionalists who perform peripheral intervention procedures in the US and Canada now…
June 26th, 2023

Bloomington, Ind. — Following the publication of an animal study examining the performance of embolization coils in arteries1 in the Journal of Vascular and Interventional Radiology (JVIR) in 2019, a second, similar study,2 published in the May 2023 issue of…
June 23rd, 2023
CAUTION: Investigational device. Limited by United States law to investigational use. Bloomington, Ind. — The U.S. Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an Investigational Device Exemption (IDE) study on the Zenith Fenestrated+ Endovascular…
June 12th, 2023

For five years, the Cook Medical headquarters in Bloomington, Indiana has hosted e-recycling events. We held it again this year, and the tradition has recently sparked other electronics recycling events at other Cook locations. It’s a part of who we…
June 2nd, 2023
Bloomington, Ind. — Cook Medical is proud to achieve a transparency partner badge from the Healthcare Industry Resiliency Collaborative (HIRC). HIRC is a nonprofit healthcare supply chain trade association. Its mission is to support supply chain best practices for better…
May 21st, 2021

Demee Koch recently shared an interview with Cook Medical and Cook Group’s president in Medium’s post, Conscious Entrepreneurship: May I introduce Pete Yonkman. Our president, Pete Yonkman, believes companies need to develop deep connections with employees, customers and communities in…
May 10th, 2021
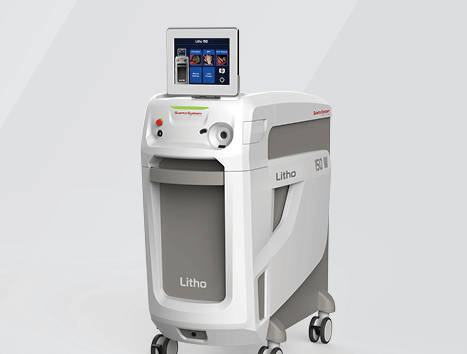
Bloomington, Ind. — The Litho 150 laser is now commercially available in the US. The product is the latest offering from Quanta System and is available through Cook Medical distribution. With power of up to 152 W, this ultra-high-power laser…
May 6th, 2021

View news coverage of this announcement below. Indianapolis, Ind. — An Indianapolis neighborhood will soon have access to fresh food with a new grocery store, brought to life through a truly unique neighborhood, corporate and non-profit collaboration. The Indy Fresh…
April 14th, 2021

West Lafayette, Ind. — The FDA has reviewed the applications to the Accreditation Scheme for Conformity Assessment (ASCA) Pilot program and has granted laboratory safety accreditation to Cook Research Incorporated (CRI), a part of Cook Medical. CRI is the nonclinical…
March 30th, 2021
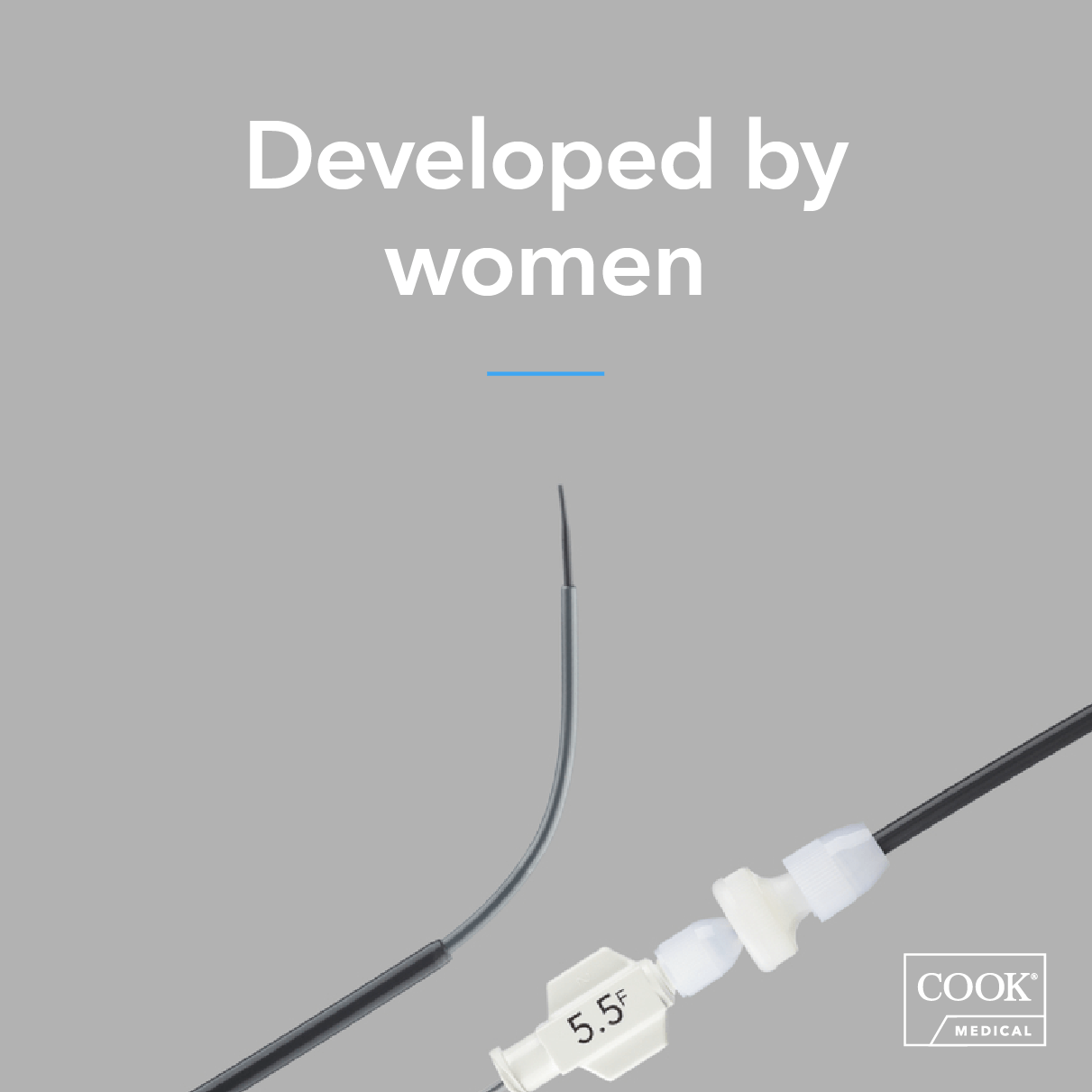
Cook Medical has a long history working with pioneers and innovators to develop products that are beneficial to both patients and clinicians. For Women’s History Month and beyond, we celebrate our current and past partnerships with women who made a difference. Product name: Rösch-Thurmond Fallopian Tube Catheterization Set…
March 2nd, 2021

Bloomington, Ind. – A new medical device manufacturing facility isn’t just bringing 100 new jobs to Indianapolis—the site will also boost the economy with a goal of contracting with 100% local minority-owned construction companies. Harmon Construction, a third-generation family-owned contractor,…
March 1st, 2021
Bloomington, Ind. — Cook Medical’s Zenith® Fenestrated+ Endovascular Graft (ZFEN+) product has received Breakthrough Device designation from the US Food and Drug Administration (FDA). This designation is granted for devices that have the potential to provide more effective treatment or…
January 11th, 2021
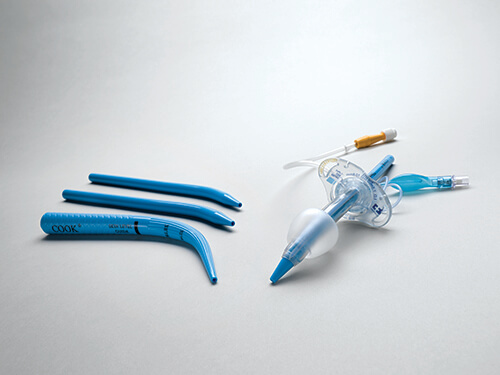
Bloomington, Ind. — Cook Medical today announced that the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays are commercially available to physicians in the United States and Canada. This product is a new iteration of the Ciaglia Blue Rhino…
January 6th, 2021
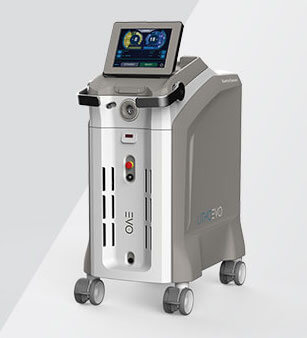
Bloomington, Ind. – The Litho EVO holmium laser is now available through Cook Medical in the United States, Austria, Germany, France, Ireland, Switzerland, and the United Kingdom. The Quanta Litho EVO laser is now available through…
June 3rd, 2020

This is a painful time in our country. It’s unfathomable that tragedies such as the deaths of George Floyd, Breonna Taylor, and Ahmaud Arbery continue to happen. Sadly, as people of color know all too well, they do happen every…
December 20th, 2019
Bloomington, Ind. — Today, Cook Medical applauds the successful efforts of legislators from the U.S. Senate and House of Representatives to repeal the medical device excise tax. “Repealing the medical device tax will help bring new life-saving devices to patients around the…
July 24th, 2018
Bloomington, Ind. – Cook is pleased with bipartisan efforts by the U.S. House of Representatives to help bring new life-saving medical devices to patients by passing H.R. 184, the Protect Medical Innovation Act. This legislation would permanently repeal the medical…
January 22nd, 2018
Bloomington, Ind. — Today, Cook Medical applauds the successful efforts of legislators in both the U.S. Senate and House of Representatives to suspend the medical device excise tax for two years. This action is a continuation of the previous two-year suspension of…
November 9th, 2017
Bloomington, Ind. – Today a jury returned a verdict in the first bellwether trial on IVC filters. An Evansville, Indiana, jury returned a unanimous verdict in favor of Cook after a three week trial. “We are pleased with this outcome.…
June 21st, 2017
The Herald-Times, a local Bloomington, Indiana paper, profiled Cook’s High School Equivalency program in a feature. Reporter Kurt Christian sat down with Cook leadership and recent program graduate Nycole to capture stories and successes of the adult education initiative. Read…
November 15th, 2016
BLOOMINGTON, Ind.-- Cook Medical has completed enrollment in the first clinical study of an iliofemoral venous stent conducted in the United States under an FDA-approved Investigational Device Exemption (IDE). The VIVO Clinical Study is a prospective, non-randomized, multi-center study intended…
December 23rd, 2015
Bloomington, Ind. — Cook Medical applauds the successful efforts of legislators in both the U.S. Senate and House of Representatives last week to suspend the burdensome medical device excise tax for two years. This action to suspend the medical device tax…
September 28th, 2015
Bloomington, Ind. — Dr. Kimihiko Kichikawa, Department of Radiology at Nara Medical University in Japan, reported two-year results of the Zilver® PTX® post-market surveillance (PMS) study on September 27, 2015, in Lisbon, Portugal. Dr. Kichikawa presented initial target data on…
June 18th, 2015
Cook is pleased that the House of Representatives, working in a bipartisan effort, has acted to help patients needing the latest medical technologies, support hospitals struggling to control healthcare expenses, and boost U.S.-based device manufacturers in a global competitive market,…
November 13th, 2013

Bloomington, Ind. — Cook Medical has today officially opened its new state-of-the-art research and development (R&D) Innovation Centre at its plant in Limerick, Ireland. The Innovation Centre together, with the expansion of cleanrooms, packaging, storage and other facilities, represents…
October 8th, 2013
Las Vegas, Nev. — Four-year data from the Zilver® PTX® Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease from Cook Medical presented today at the 2013 Vascular Interventional Advances (VIVA) meeting demonstrates 75 percent primary patency in the superficial femoral…
April 2nd, 2013
Bloomington, Ind.— Cook Group officials today acknowledged an Indiana General Assembly concurrent resolution sponsored by State Representative Bob Heaton extending "extreme gratitude" for the company’s 50-year history and its beneficial impact on Indiana’s economy and culture. "On behalf of the Cook family,…
March 22nd, 2013
Bloomington, Ind. — Cook Medical is gratified and U.S. patients are thankful for the bipartisan vote in the U.S. Senate on Thursday that overwhelmingly passed the Hatch-Klobuchar amendment to the Senate Budget Resolution to repeal the 2.3 percent tax on medical…
October 22nd, 2012
Bloomington, Ind. — The Grant Street Inn has opened a new 16-room addition. The new building was designed and constructed to meet Leadership in Energy and Environmental Design (LEED) certification requirements. The facility will be the fourth LEED-certified building in Bloomington,…
September 28th, 2012
Baesweiler, North Rhine-Westphalia — Cook Medical, a world leader in minimally invasive medical technologies, today announced a major development in the extension of its European operations with the opening of a new €15m distribution center in Baesweiler, Germany. Located less than…
September 10th, 2012
Washington, D.C. — Cook Medical, a world leader in minimally invasive medical device technology, has launched its new Otolaryngology/Head and Neck Surgery (OHNS) clinical division to bring the benefits of the company’s devices for non-surgical procedures to a new group…
June 28th, 2012
Bloomington, Ind. – With the U.S. Supreme Court ruling upholding the constitutionality of the Affordable Care Act (ACA), Cook Medical now calls for the U.S. Senate to repeal the 2.3 percent medical device excise tax included in that legislation. “The…
June 4th, 2012
Bloomington, Ind. – This is an important week for employees and businesses across Indiana and our nation. The Committee on Ways and Means in the U.S. House of Representatives, with support from both parties, voted 23-11 to approve legislation, H.R.…
April 9th, 2012
Bloomington, Ind. — As part of its ongoing commitment to patients needing emerging new cellular therapies, Cook Group has acquired the assets of General BioTechnology LLC and launched a new company, Cook General BioTechnology LLC (CGBT). Based in Indianapolis, CGBT…
August 19th, 2011
Bloomington, Ind. – Cook Incorporated, a global leader in endovascular technologies, announced today that Judge Tanya Walton Pratt of the United States District Court for the Southern District of Indiana in Indianapolis has issued a Markman ruling in the ongoing…
April 15th, 2011
Bloomington, Ind. – William Alfred Cook, founder of the Cook Group global network of companies and a pioneer in the development of life-saving minimally invasive medical device technology, died Friday at approximately 4:30 p.m. EDT at his Bloomington home of congestive…
 This article is part of our Ask the CMO series, where Cook Medical’s chief medical officer, Dr. John Kaufman, answers questions. Learn more about Dr. Kaufman in his Meet Our Leaders bio. Q: You have worked with many other medical device companies and many…
This article is part of our Ask the CMO series, where Cook Medical’s chief medical officer, Dr. John Kaufman, answers questions. Learn more about Dr. Kaufman in his Meet Our Leaders bio. Q: You have worked with many other medical device companies and many…  This article is part of our Ask the CMO series, where Cook Medical’s chief medical officer, Dr. John Kaufman, answers questions. Learn more about Dr. Kaufman in his Meet Our Leaders bio. Q: You have worked with many other medical device companies and many…
This article is part of our Ask the CMO series, where Cook Medical’s chief medical officer, Dr. John Kaufman, answers questions. Learn more about Dr. Kaufman in his Meet Our Leaders bio. Q: You have worked with many other medical device companies and many…  Cook Medical has announced a new partnership with Mixxer Community Makerspace to bring more opportunities, more creativity, and more “I-can’t-believe-I-built-this” moments for people across the Triad. Through the new partnership, Cook will provide the resources to welcome even more creators…
Cook Medical has announced a new partnership with Mixxer Community Makerspace to bring more opportunities, more creativity, and more “I-can’t-believe-I-built-this” moments for people across the Triad. Through the new partnership, Cook will provide the resources to welcome even more creators…  Cook Medical and Siemens Healthineers today announced a strategic commercial partnership aimed at setting a new standard for interventional medicine. The collaboration combines the power of Siemens Healthineers real-time magnetic resonance imaging with Cook Medical's deep interventional procedure expertise, along…
Cook Medical and Siemens Healthineers today announced a strategic commercial partnership aimed at setting a new standard for interventional medicine. The collaboration combines the power of Siemens Healthineers real-time magnetic resonance imaging with Cook Medical's deep interventional procedure expertise, along…  L-R: Rick Simms (Cook Medical, National Manager, GPO Account Executives), Amber Pastorek (Cook Medical, Account Executive Manager), Glenn Coleman (Premier, Chief Financial Officer & Chief Administrative Officer), Bob Stanley (Cook Medical, National GPO Account Executive), Brian…
L-R: Rick Simms (Cook Medical, National Manager, GPO Account Executives), Amber Pastorek (Cook Medical, Account Executive Manager), Glenn Coleman (Premier, Chief Financial Officer & Chief Administrative Officer), Bob Stanley (Cook Medical, National GPO Account Executive), Brian…  Bloomington, Ind. — Cook Medical has been awarded a contract from Vizient for our portfolio of urology devices. This agreement provides increased access to…
Bloomington, Ind. — Cook Medical has been awarded a contract from Vizient for our portfolio of urology devices. This agreement provides increased access to…  Cook Medical, a global medical device manufacturer, has announced a new collaboration to incorporate DuPont™ Tyvek® with Renewable Attribution into its medical device packaging. Together with Nelipak® Healthcare Packaging, an Authorized Converter of Tyvek® Healthcare Packaging products, Cook is taking…
Cook Medical, a global medical device manufacturer, has announced a new collaboration to incorporate DuPont™ Tyvek® with Renewable Attribution into its medical device packaging. Together with Nelipak® Healthcare Packaging, an Authorized Converter of Tyvek® Healthcare Packaging products, Cook is taking… 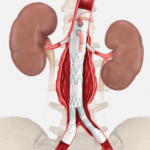 CAUTION - INVESTIGATIONAL DEVICE. Limited by United States law to investigational use. Bloomington, Ind. — Cook Medical has enrolled the final patient in the global clinical study of the ZENITH® FENESTRATED+ Endovascular…
CAUTION - INVESTIGATIONAL DEVICE. Limited by United States law to investigational use. Bloomington, Ind. — Cook Medical has enrolled the final patient in the global clinical study of the ZENITH® FENESTRATED+ Endovascular…  CAUTION: Investigational Device. Limited by United States law to investigational use. Bloomington, Ind. — The U.S. Food and Drug Administration (FDA) has granted approval…
CAUTION: Investigational Device. Limited by United States law to investigational use. Bloomington, Ind. — The U.S. Food and Drug Administration (FDA) has granted approval…  We are passionate about innovation in the supply chain industry. Our goal is to connect with and listen to healthcare professionals to identify unmet needs and use our expertise to imagine new solutions. For years, we have consistently heard that…
We are passionate about innovation in the supply chain industry. Our goal is to connect with and listen to healthcare professionals to identify unmet needs and use our expertise to imagine new solutions. For years, we have consistently heard that…  Leveraging Cook's history of innovative medical devices in a new procedural setting Bloomington, Ind. — Cook Medical today announced the launch of its new Interventional Magnetic Resonance Imaging (iMRI) division, marking a significant step forward in its commitment to transforming…
Leveraging Cook's history of innovative medical devices in a new procedural setting Bloomington, Ind. — Cook Medical today announced the launch of its new Interventional Magnetic Resonance Imaging (iMRI) division, marking a significant step forward in its commitment to transforming…  Cook Medical has released its Social Impact & Sustainability Report for 2024, highlighting the company’s commitment to working sustainably and responsibly while fulfilling its purpose to improve the lives of everyone it serves. The report reflects on the achievements of Cook's teams across…
Cook Medical has released its Social Impact & Sustainability Report for 2024, highlighting the company’s commitment to working sustainably and responsibly while fulfilling its purpose to improve the lives of everyone it serves. The report reflects on the achievements of Cook's teams across…  Bloomington, Ind. — Cook Medical’s Zenith® Iliac Branch Device (ZBIS) is now commercially available in the United States with FDA approval as an endovascular treatment option for aortoiliac or…
Bloomington, Ind. — Cook Medical’s Zenith® Iliac Branch Device (ZBIS) is now commercially available in the United States with FDA approval as an endovascular treatment option for aortoiliac or…  Bloomington, Ind. — Cook Medical announced a collaboration with Mendaera™ Inc., a Silicon Valley-based healthcare technology company. The goal of this collaboration is to create cutting-edge solutions for needle-based interventions in urological procedures by combining Cook's market-leading needles with Mendaera's handheld…
Bloomington, Ind. — Cook Medical announced a collaboration with Mendaera™ Inc., a Silicon Valley-based healthcare technology company. The goal of this collaboration is to create cutting-edge solutions for needle-based interventions in urological procedures by combining Cook's market-leading needles with Mendaera's handheld…  Bloomington, Ind. — Cook Medical’s Zilver PTX drug-eluting stent (DES) has lower rates of in-stent occlusions among patients with restenosis at three years than Boston Scientific’s Eluvia DES, according to real-world data from the REALDES study. The data, published by…
Bloomington, Ind. — Cook Medical’s Zilver PTX drug-eluting stent (DES) has lower rates of in-stent occlusions among patients with restenosis at three years than Boston Scientific’s Eluvia DES, according to real-world data from the REALDES study. The data, published by…  Our mission stays the same, but at Cook, we’re relentlessly inventive and proactively adapting to the needs of physicians and patients. We previously shared three ways Cook is changing. Since then, we’ve made other significant advancements. Here are four more…
Our mission stays the same, but at Cook, we’re relentlessly inventive and proactively adapting to the needs of physicians and patients. We previously shared three ways Cook is changing. Since then, we’ve made other significant advancements. Here are four more…  Bloomington, Ind. — Cook Medical is now offering Magneto technology on the Quanta System 100W and 150W High Power Ho:YAG Laser Systems. Magneto technology allows physicians to perform a wider array of endourological procedures than ever before with one laser…
Bloomington, Ind. — Cook Medical is now offering Magneto technology on the Quanta System 100W and 150W High Power Ho:YAG Laser Systems. Magneto technology allows physicians to perform a wider array of endourological procedures than ever before with one laser…  Bloomington, Ind. — Cook Medical’s Zenith Alpha® 2 Thoracic Endovascular Graft (ZTA2) is now commercially available throughout the United States. ZTA2 is the first aortic device on the market that has evolved to accommodate CO2 flushing and has an approved…
Bloomington, Ind. — Cook Medical’s Zenith Alpha® 2 Thoracic Endovascular Graft (ZTA2) is now commercially available throughout the United States. ZTA2 is the first aortic device on the market that has evolved to accommodate CO2 flushing and has an approved…  Please join us as we highlight some of our leaders, learn about their experiences, and hear how they are talking about the positive changes we’re making to meet our vision of the future at Cook. Follow along for the full Meet…
Please join us as we highlight some of our leaders, learn about their experiences, and hear how they are talking about the positive changes we’re making to meet our vision of the future at Cook. Follow along for the full Meet…  Bloomington, Ind. — Furthering their commitment to relentlessly delivering new products and services to address unmet customer needs, today Cook Medical announced a strategic investment in the urology space. Zenflow is a medical device company developing a minimally invasive treatment…
Bloomington, Ind. — Furthering their commitment to relentlessly delivering new products and services to address unmet customer needs, today Cook Medical announced a strategic investment in the urology space. Zenflow is a medical device company developing a minimally invasive treatment…  Bloomington, Ind. — Cook Medical is now distributing the PillSense™ GI Bleed Detection System in the United States. PillSense™ is a novel device that can detect upper gastrointestinal (GI) bleeding in patients in under 10 minutes. Cook is constantly changing…
Bloomington, Ind. — Cook Medical is now distributing the PillSense™ GI Bleed Detection System in the United States. PillSense™ is a novel device that can detect upper gastrointestinal (GI) bleeding in patients in under 10 minutes. Cook is constantly changing…  Bloomington, Ind. — Cook Medical is now offering the NestVT Vitrification Device in the United States. This device is a secure solution for embryos and oocytes during vitrification, cryo-storage and relocation. Along with the launch of this innovative product, Cook…
Bloomington, Ind. — Cook Medical is now offering the NestVT Vitrification Device in the United States. This device is a secure solution for embryos and oocytes during vitrification, cryo-storage and relocation. Along with the launch of this innovative product, Cook…  Bloomington, Ind. — Cook Medical announced this morning it has signed an agreement with Merit Medical to purchase Cook’s Lead Management portfolio. This announcement is the conclusion of a series of planned Cook divestures across multiple product lines and specialties…
Bloomington, Ind. — Cook Medical announced this morning it has signed an agreement with Merit Medical to purchase Cook’s Lead Management portfolio. This announcement is the conclusion of a series of planned Cook divestures across multiple product lines and specialties…  Bloomington, Ind. — Cook Medical announced this morning it has signed a letter of intent with Astorg, a leading private equity firm with an extensive and successful track record in global healthcare investments, to purchase Cook’s Reproductive Health business (“Cook…
Bloomington, Ind. — Cook Medical announced this morning it has signed a letter of intent with Astorg, a leading private equity firm with an extensive and successful track record in global healthcare investments, to purchase Cook’s Reproductive Health business (“Cook…  Bloomington, Ind. — Ross Whittaker, a manager of manufacturing engineering at Cook, was named a member of the Conexus Rising 30 Class of 2024 for his exceptional work in advanced manufacturing and logistics (AML) and the changes he and his…
Bloomington, Ind. — Ross Whittaker, a manager of manufacturing engineering at Cook, was named a member of the Conexus Rising 30 Class of 2024 for his exceptional work in advanced manufacturing and logistics (AML) and the changes he and his…  At Cook, our goal has always been to provide devices and services to support minimally invasive procedures for patients. That hasn’t changed. However, what has changed is the way we work toward that goal. In November 2023, we announced an…
At Cook, our goal has always been to provide devices and services to support minimally invasive procedures for patients. That hasn’t changed. However, what has changed is the way we work toward that goal. In November 2023, we announced an…  Bloomington, Ind. — In her more than 15 years of experience at Cook Medical, Amy Tyrrell has always prioritized relationships—both inside and outside the company. Her hard work over the years was recognized when she earned the Captis Medical Device…
Bloomington, Ind. — In her more than 15 years of experience at Cook Medical, Amy Tyrrell has always prioritized relationships—both inside and outside the company. Her hard work over the years was recognized when she earned the Captis Medical Device…  Cook Medical has released its Social Impact & Sustainability Report for 2023, highlighting the company’s commitment to working sustainably and responsibly across all of its businesses while fulfilling its purpose to improve the lives of everyone it serves. The report…
Cook Medical has released its Social Impact & Sustainability Report for 2023, highlighting the company’s commitment to working sustainably and responsibly across all of its businesses while fulfilling its purpose to improve the lives of everyone it serves. The report…  Cook Medical’s Beacon® Tip Sizing Catheters are now available in the US and Canada. The Beacon Tip Sizing Catheter is available in a variety of lengths and tip configurations, empowering physicians to treat patients with the best fitting devices for…
Cook Medical’s Beacon® Tip Sizing Catheters are now available in the US and Canada. The Beacon Tip Sizing Catheter is available in a variety of lengths and tip configurations, empowering physicians to treat patients with the best fitting devices for…  An Interview with Cook chief medical officer Dr. John Kaufman About 20 years ago, Cook started working on a venous valve. Our goal was to create a long-term solution for patients suffering from chronic venous insufficiency (CVI) due to reflux…
An Interview with Cook chief medical officer Dr. John Kaufman About 20 years ago, Cook started working on a venous valve. Our goal was to create a long-term solution for patients suffering from chronic venous insufficiency (CVI) due to reflux…  Bloomington, Ind. — Cook Medical and EnteraSense, Ltd. have entered into an agreement where Cook will become the exclusive distributor of EnteraSense’s…
Bloomington, Ind. — Cook Medical and EnteraSense, Ltd. have entered into an agreement where Cook will become the exclusive distributor of EnteraSense’s…  Bloomington, Ind. — Cook Medical launched the next-generation EchoTip ClearCore™ Endoscopic Ultrasound (EUS) Biopsy Needle in the United States. As Cook changes to better serve our customers, we look forward to developing and offering products for a wider variety of…
Bloomington, Ind. — Cook Medical launched the next-generation EchoTip ClearCore™ Endoscopic Ultrasound (EUS) Biopsy Needle in the United States. As Cook changes to better serve our customers, we look forward to developing and offering products for a wider variety of…  Bloomington, Ind. — Cook Medical is now offering the Cook Medical's Ascend™ Single-Use Flexible Ureteroscope in the United States and Canada. This addition is a positive change that will empower Cook to better serve more urology customers with a complete…
Bloomington, Ind. — Cook Medical is now offering the Cook Medical's Ascend™ Single-Use Flexible Ureteroscope in the United States and Canada. This addition is a positive change that will empower Cook to better serve more urology customers with a complete…  Bloomington, Ind. — While Marsha Lovejoy has been leading public relations at Cook Medical, she’s also been dedicating her time to community service and advocacy and…
Bloomington, Ind. — While Marsha Lovejoy has been leading public relations at Cook Medical, she’s also been dedicating her time to community service and advocacy and…  Bloomington, IN and Merrimack, NH—Today, Getinge and Cook Medical announced an exclusive sales and distribution agreement for the iCast® covered stent system, which recently received premarket approval for treatment of symptomatic iliac arterial occlusive disease. Cook Medical will assume…
Bloomington, IN and Merrimack, NH—Today, Getinge and Cook Medical announced an exclusive sales and distribution agreement for the iCast® covered stent system, which recently received premarket approval for treatment of symptomatic iliac arterial occlusive disease. Cook Medical will assume…  Bloomington, Ind. — Today, Cook Medical and Bentley announced a United States distribution agreement for the BeBack Catheter. Over the coming months, Cook Medical will be assuming commercial responsibilities for this Bentley product. “We are excited to welcome Bentley’s BeBack…
Bloomington, Ind. — Today, Cook Medical and Bentley announced a United States distribution agreement for the BeBack Catheter. Over the coming months, Cook Medical will be assuming commercial responsibilities for this Bentley product. “We are excited to welcome Bentley’s BeBack… 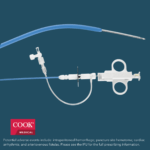 Bloomington, Ind. — Cook Medical’s shorter Liver Access and Biopsy Set (LABS) is back on the US market with a new pediatric indication. Originally indicated for use in adults only, the set now has FDA clearance for use in adolescents,…
Bloomington, Ind. — Cook Medical’s shorter Liver Access and Biopsy Set (LABS) is back on the US market with a new pediatric indication. Originally indicated for use in adults only, the set now has FDA clearance for use in adolescents,… 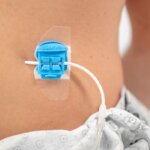 Bloomington, Ind. and Belgium — Cook Medical, a global medical device manufacturer, has partnered with Bedal International, a company specializing in catheter securement devices marketed under the brand FlexGRIP®. FlexGRIP devices are now available as an addition to Cook’s comprehensive…
Bloomington, Ind. and Belgium — Cook Medical, a global medical device manufacturer, has partnered with Bedal International, a company specializing in catheter securement devices marketed under the brand FlexGRIP®. FlexGRIP devices are now available as an addition to Cook’s comprehensive… 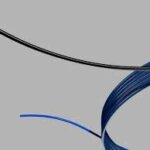 Bloomington, Ind. — Cook Medical’s Motion Hybrid Wire Guide is now commercially available in Canada. This two-in-one wire guide is an excellent choice for clinicians in urological specialties because it functions as both an access wire guide and a safety…
Bloomington, Ind. — Cook Medical’s Motion Hybrid Wire Guide is now commercially available in Canada. This two-in-one wire guide is an excellent choice for clinicians in urological specialties because it functions as both an access wire guide and a safety… 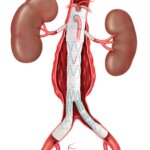 CAUTION - INVESTIGATIONAL DEVICE. Limited by United States law to investigational use. Bloomington, Ind. — Cook Medical today announced the first patient treated in the clinical study of the ZENITH® FENESTRATED+ Endovascular Graft (ZFEN+) in the United States. The procedure was performed…
CAUTION - INVESTIGATIONAL DEVICE. Limited by United States law to investigational use. Bloomington, Ind. — Cook Medical today announced the first patient treated in the clinical study of the ZENITH® FENESTRATED+ Endovascular Graft (ZFEN+) in the United States. The procedure was performed…  Bloomington, Ind. — Cook Medical today announced the sale of the remaining Otolaryngology, Head & Neck Surgery (OHNS) product lines, in the latest of a series of strategic decisions aligned to its 5-year vision. Cook signed an agreement with C2Dx,…
Bloomington, Ind. — Cook Medical today announced the sale of the remaining Otolaryngology, Head & Neck Surgery (OHNS) product lines, in the latest of a series of strategic decisions aligned to its 5-year vision. Cook signed an agreement with C2Dx,…  Bloomington, Ind. — Cook Medical’s Slip-Cath® Beacon® Tip Hydrophilic Selective Catheter is now available for use in the U.S. and Canada. Slip-Cath…
Bloomington, Ind. — Cook Medical’s Slip-Cath® Beacon® Tip Hydrophilic Selective Catheter is now available for use in the U.S. and Canada. Slip-Cath…  Bloomington, Ind. — Cook Medical won the 2023 Loyal Partner Supplier Award from Capstone Health Alliance for outstanding work from Cook’s MedSurg division. Cook is proud…
Bloomington, Ind. — Cook Medical won the 2023 Loyal Partner Supplier Award from Capstone Health Alliance for outstanding work from Cook’s MedSurg division. Cook is proud…  The last several years have brought significant change to Cook. To ensure that we are positioned to be successful in this new world, our global leadership teams have implemented a new vision and strategic plan that were informed by extensive…
The last several years have brought significant change to Cook. To ensure that we are positioned to be successful in this new world, our global leadership teams have implemented a new vision and strategic plan that were informed by extensive…  Bloomington, Ind. — The US Department of Veterans Affairs (VA) has awarded Cook Medical a Next Generation Implant Contract for implanted medical devices. This contract will streamline the procurement process for the VA supply chain team. This contract is another…
Bloomington, Ind. — The US Department of Veterans Affairs (VA) has awarded Cook Medical a Next Generation Implant Contract for implanted medical devices. This contract will streamline the procurement process for the VA supply chain team. This contract is another…  Bloomington, Ind. — Cook Medical is pleased to promote Barry Slowey to vice president and chief sustainability officer. His new position was effective September 8, 2023. In his new appointment, Barry will provide leadership and implement the goals of Cook’s Social…
Bloomington, Ind. — Cook Medical is pleased to promote Barry Slowey to vice president and chief sustainability officer. His new position was effective September 8, 2023. In his new appointment, Barry will provide leadership and implement the goals of Cook’s Social…  Bloomington, Ind. — At the 2023 Vizient Connections Summit, Cook Medical won the Vizient Supply Assurance Supplier of the Year Award. This award demonstrates Cook’s transparency and commitment to communication during the supply chain process with healthcare organizations that are…
Bloomington, Ind. — At the 2023 Vizient Connections Summit, Cook Medical won the Vizient Supply Assurance Supplier of the Year Award. This award demonstrates Cook’s transparency and commitment to communication during the supply chain process with healthcare organizations that are…  Bloomington, Ind. — Cook Medical is pleased to promote Amber Beauchamp to vice president, global human resources at Cook Group and Cook Medical effective September 1, 2023. Beauchamp has more than 17 years of HR experience in multiple industries. She…
Bloomington, Ind. — Cook Medical is pleased to promote Amber Beauchamp to vice president, global human resources at Cook Group and Cook Medical effective September 1, 2023. Beauchamp has more than 17 years of HR experience in multiple industries. She…  Bringing the latest in medical technologies to the youngest patients INDIANAPOLIS and WEST LAFAYETTE, Ind. – Purdue University, the Indiana University School of Medicine and medical device company Cook Medical are focusing a new alliance on clinical needs in pediatrics…
Bringing the latest in medical technologies to the youngest patients INDIANAPOLIS and WEST LAFAYETTE, Ind. – Purdue University, the Indiana University School of Medicine and medical device company Cook Medical are focusing a new alliance on clinical needs in pediatrics…  Bloomington, Ind. — As announced at the 2023 Vascular Annual Meeting (VAM), the Advance Serenity® Hydrophilic PTA Balloon Catheter product line is now available with even more options. Interventionalists who perform peripheral intervention procedures in the US and Canada now…
Bloomington, Ind. — As announced at the 2023 Vascular Annual Meeting (VAM), the Advance Serenity® Hydrophilic PTA Balloon Catheter product line is now available with even more options. Interventionalists who perform peripheral intervention procedures in the US and Canada now…  For five years, the Cook Medical headquarters in Bloomington, Indiana has hosted e-recycling events. We held it again this year, and the tradition has recently sparked other electronics recycling events at other Cook locations. It’s a part of who we…
For five years, the Cook Medical headquarters in Bloomington, Indiana has hosted e-recycling events. We held it again this year, and the tradition has recently sparked other electronics recycling events at other Cook locations. It’s a part of who we…  Bloomington, Ind. — Cook Medical is proud to achieve a transparency partner badge from the Healthcare Industry Resiliency Collaborative (HIRC). HIRC is a nonprofit healthcare supply chain trade association. Its mission is to support supply chain best practices for better…
Bloomington, Ind. — Cook Medical is proud to achieve a transparency partner badge from the Healthcare Industry Resiliency Collaborative (HIRC). HIRC is a nonprofit healthcare supply chain trade association. Its mission is to support supply chain best practices for better…  The email below, along with a video from our president and an FAQ document, was sent to all Cook Medical employees on Monday, May 15 at 1pm EST. Memo header: Company announcement: A difficult step for our strategic success…
The email below, along with a video from our president and an FAQ document, was sent to all Cook Medical employees on Monday, May 15 at 1pm EST. Memo header: Company announcement: A difficult step for our strategic success…  Bloomington, Ind. — For the third year in a row, Cook Medical has received a Greenovation award from Kimberly-Clark’s RightCycle Program. The award is for Cook’s continued sustainability leadership and participation in a landfill diversion program for nitrile gloves. [caption…
Bloomington, Ind. — For the third year in a row, Cook Medical has received a Greenovation award from Kimberly-Clark’s RightCycle Program. The award is for Cook’s continued sustainability leadership and participation in a landfill diversion program for nitrile gloves. [caption…  Jennifer Ludden and Marisa Peñaloza shared Cook Medical's housing initiative on NPR's Morning Edition. The piece is called "Would you live next to co-workers for the right price? This company is betting yes." NPR: Listen here On Tuesday, May 2,…
Jennifer Ludden and Marisa Peñaloza shared Cook Medical's housing initiative on NPR's Morning Edition. The piece is called "Would you live next to co-workers for the right price? This company is betting yes." NPR: Listen here On Tuesday, May 2,…  Bloomington, Ind. — Cook Medical announces the first patient treated in a clinical study to evaluate a new venous valve designed for treating chronic venous insufficiency. The patient was treated by Dr. Mauricio Alviar, vascular surgeon and principal investigator, of…
Bloomington, Ind. — Cook Medical announces the first patient treated in a clinical study to evaluate a new venous valve designed for treating chronic venous insufficiency. The patient was treated by Dr. Mauricio Alviar, vascular surgeon and principal investigator, of…  Bloomington, Ind. — Cook Medical has received a contract with Vizient for endoscopy devices. This new agreement will allow Cook to continue to offer our endoscopy devices at negotiated pricing terms to healthcare facilities that are members of Vizient. With…
Bloomington, Ind. — Cook Medical has received a contract with Vizient for endoscopy devices. This new agreement will allow Cook to continue to offer our endoscopy devices at negotiated pricing terms to healthcare facilities that are members of Vizient. With…  Cook Medical aims to raise awareness around head and neck cancer and dysphagia through Dale Maloney’s story According to the Yale School of Medicine, head and neck cancer affected over 46,000 people last year.1 In addition, a study conducted in…
Cook Medical aims to raise awareness around head and neck cancer and dysphagia through Dale Maloney’s story According to the Yale School of Medicine, head and neck cancer affected over 46,000 people last year.1 In addition, a study conducted in…  Meredith Hackler, a reporter with WRTV, recently shared a story about Cook Medical on WRTV’s Hiring Hoosiers called “Workforce pilot program will aim to hire more people with disabilities.” WRTV, a news station local to Indianapolis, featured a story on…
Meredith Hackler, a reporter with WRTV, recently shared a story about Cook Medical on WRTV’s Hiring Hoosiers called “Workforce pilot program will aim to hire more people with disabilities.” WRTV, a news station local to Indianapolis, featured a story on…  Solar arrays brighten a sustainable future at Cook’s headquarters They traveled by steam ship and by semi-trailer truck across the globe to settle in Bloomington, Indiana. The journey began in Kolkata, India. Over 3,000 solar panels were carefully bundled in…
Solar arrays brighten a sustainable future at Cook’s headquarters They traveled by steam ship and by semi-trailer truck across the globe to settle in Bloomington, Indiana. The journey began in Kolkata, India. Over 3,000 solar panels were carefully bundled in…  Bloomington, Ind. — Cook Medical has launched a new, streamlined portfolio of urological bipolar electrodes in the U.S. This portfolio includes the products that urologists use most frequently to focus on daily electrode needs when performing procedures on the bladder…
Bloomington, Ind. — Cook Medical has launched a new, streamlined portfolio of urological bipolar electrodes in the U.S. This portfolio includes the products that urologists use most frequently to focus on daily electrode needs when performing procedures on the bladder…  Erik L. helps Cook share knowledge with global decision-makers and thought leaders in the field of health economics Erik L. is the Health Economics manager for Cook in the United States, and his team is part of the Health Economics…
Erik L. helps Cook share knowledge with global decision-makers and thought leaders in the field of health economics Erik L. is the Health Economics manager for Cook in the United States, and his team is part of the Health Economics…  Raised to help others, Cook employee honors his mother in the most charitable way Jeff P. started at Cook Medical in 2014 as an Endoscopy district manager for the San Diego territory. After six years, he was promoted to Market…
Raised to help others, Cook employee honors his mother in the most charitable way Jeff P. started at Cook Medical in 2014 as an Endoscopy district manager for the San Diego territory. After six years, he was promoted to Market…  BLOOMINGTON, Ind. — It its inaugural HR Impact Awards, the Indianapolis Business Journal recognized Cook Medical’s High School Equivalency program. Cook’s program won in the Training and Development category of the HR Impact awards, a category that recognizes programs that help…
BLOOMINGTON, Ind. — It its inaugural HR Impact Awards, the Indianapolis Business Journal recognized Cook Medical’s High School Equivalency program. Cook’s program won in the Training and Development category of the HR Impact awards, a category that recognizes programs that help…  Davis P. and Alex B. already had a couple of things in common—they’re both Indianapolis natives and both work in Cook’s MedSurg Division. This spring they added a third thing to that list when it was announced that they had…
Davis P. and Alex B. already had a couple of things in common—they’re both Indianapolis natives and both work in Cook’s MedSurg Division. This spring they added a third thing to that list when it was announced that they had…  Bloomington, Ind. — About 3,000 solar panels now line the rooftops at Cook Medical’s headquarters. This project to invest in sustainable solar energy is Cook’s most recent initiative to create cleaner, healthier communities. At the beginning of 2022, Cook Medical…
Bloomington, Ind. — About 3,000 solar panels now line the rooftops at Cook Medical’s headquarters. This project to invest in sustainable solar energy is Cook’s most recent initiative to create cleaner, healthier communities. At the beginning of 2022, Cook Medical…  Bloomington, Ind. — Two Cook Medical managers were nominated for and selected as recipients of this year’s 23 Pair Awards hosted by BioCrossroads. Jillian Ivers and Lauren Manges lead teams at Cook and have been identified as outstanding emerging talents…
Bloomington, Ind. — Two Cook Medical managers were nominated for and selected as recipients of this year’s 23 Pair Awards hosted by BioCrossroads. Jillian Ivers and Lauren Manges lead teams at Cook and have been identified as outstanding emerging talents…  Indianapolis, Ind. – The Indy Fresh Market will have a one-time economic impact totaling $11.1 million, plus an annual impact of $4.6 million in wages and benefits, and related spending. An estimated 39 direct jobs and 61 full-time equivalent jobs will…
Indianapolis, Ind. – The Indy Fresh Market will have a one-time economic impact totaling $11.1 million, plus an annual impact of $4.6 million in wages and benefits, and related spending. An estimated 39 direct jobs and 61 full-time equivalent jobs will…  The lockdown in Shanghai and the employees who helped by moving in to the facility Editor's note: Shanghai has begun lifting a strict, two-month lockdown that was designed to contain rising Covid cases. We want to share an interesting…
The lockdown in Shanghai and the employees who helped by moving in to the facility Editor's note: Shanghai has begun lifting a strict, two-month lockdown that was designed to contain rising Covid cases. We want to share an interesting…  Artist Amiah Mims brings murals to life at the 38th and Sheridan facility Editor’s note: We believe that it’s possible to do good business while doing good in our communities. We’re proud to work in collaboration with partners on a…
Artist Amiah Mims brings murals to life at the 38th and Sheridan facility Editor’s note: We believe that it’s possible to do good business while doing good in our communities. We’re proud to work in collaboration with partners on a…  Bloomington, Ind. — Cook Medical has received a Chelsea Santucci Greenovation Award from Kimberly-Clark Professional. Cook won this award for its industry-leading efforts in recycling nitrile gloves. Cook Medical was recognized for participating in The RightCycle Program, the first large-scale…
Bloomington, Ind. — Cook Medical has received a Chelsea Santucci Greenovation Award from Kimberly-Clark Professional. Cook won this award for its industry-leading efforts in recycling nitrile gloves. Cook Medical was recognized for participating in The RightCycle Program, the first large-scale… 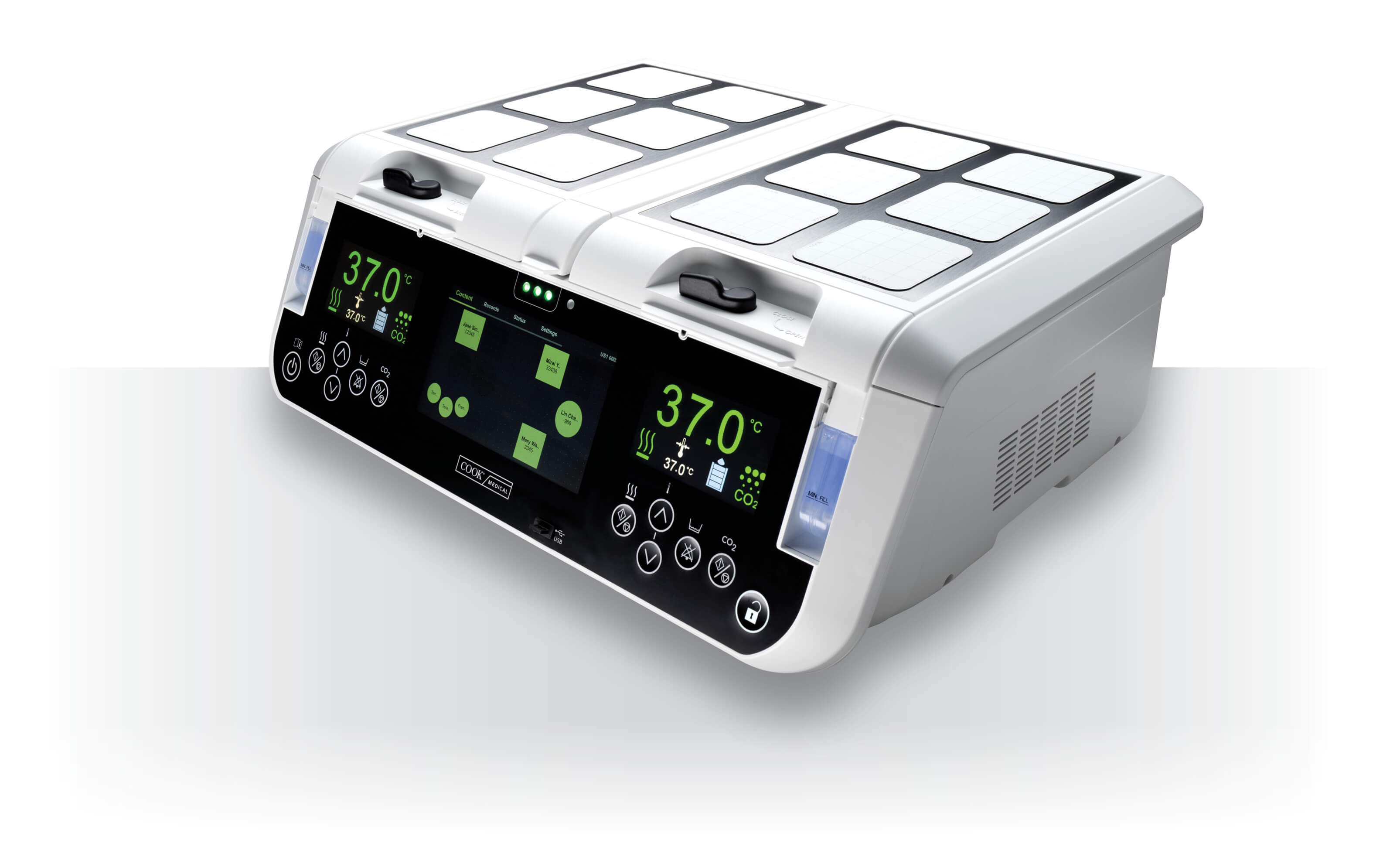 Bloomington, Ind. — The MINC+™ Benchtop Incubator is now available to clinical embryologists and IVF clinics in the US and Canada. The MINC+ is the next generation of the MINC® Mini Incubator, a benchtop incubator that has been a standard…
Bloomington, Ind. — The MINC+™ Benchtop Incubator is now available to clinical embryologists and IVF clinics in the US and Canada. The MINC+ is the next generation of the MINC® Mini Incubator, a benchtop incubator that has been a standard…  Bloomington, Ind. — Two Cook Medical employees applied and were selected for a fellowship from the Mitch Daniels Leadership Foundation (MDLF). Davis Payton and Alex Brethauer, both managers at Cook Medical, have shown exceptional commitment to innovation and investment in…
Bloomington, Ind. — Two Cook Medical employees applied and were selected for a fellowship from the Mitch Daniels Leadership Foundation (MDLF). Davis Payton and Alex Brethauer, both managers at Cook Medical, have shown exceptional commitment to innovation and investment in…  Today’s ribbon cutting ceremony marks a new beginning in a community that was once forgotten. Indianapolis, Ind. — A unique partnership between neighbors, a medical device company, government and non-profits today marks an important milestone today at 38th and Sheridan…
Today’s ribbon cutting ceremony marks a new beginning in a community that was once forgotten. Indianapolis, Ind. — A unique partnership between neighbors, a medical device company, government and non-profits today marks an important milestone today at 38th and Sheridan…  There was no easing into their workday for Susan S. and the rest of the nursing staff in the COVID-19 intensive care unit (ICU) at their hospital in Copenhagen, Denmark. At the beginning of every shift, they would gather to…
There was no easing into their workday for Susan S. and the rest of the nursing staff in the COVID-19 intensive care unit (ICU) at their hospital in Copenhagen, Denmark. At the beginning of every shift, they would gather to…  Winston-Salem, N.C. — Cook Medical has always been committed to improving people’s lives. That promise spans from patient care to the communities in which the company operates and beyond. Today Cook Medical’s Winston-Salem manufacturing facility announced that they have recently…
Winston-Salem, N.C. — Cook Medical has always been committed to improving people’s lives. That promise spans from patient care to the communities in which the company operates and beyond. Today Cook Medical’s Winston-Salem manufacturing facility announced that they have recently…  View news coverage of this announcement below. BLOOMINGTON, Ind. — Intended to serve middle-income workers, workforce housing is in short supply in communities across south-central Indiana, especially during the current housing price spike. After hearing from employees and surrounding communities, Cook…
View news coverage of this announcement below. BLOOMINGTON, Ind. — Intended to serve middle-income workers, workforce housing is in short supply in communities across south-central Indiana, especially during the current housing price spike. After hearing from employees and surrounding communities, Cook… 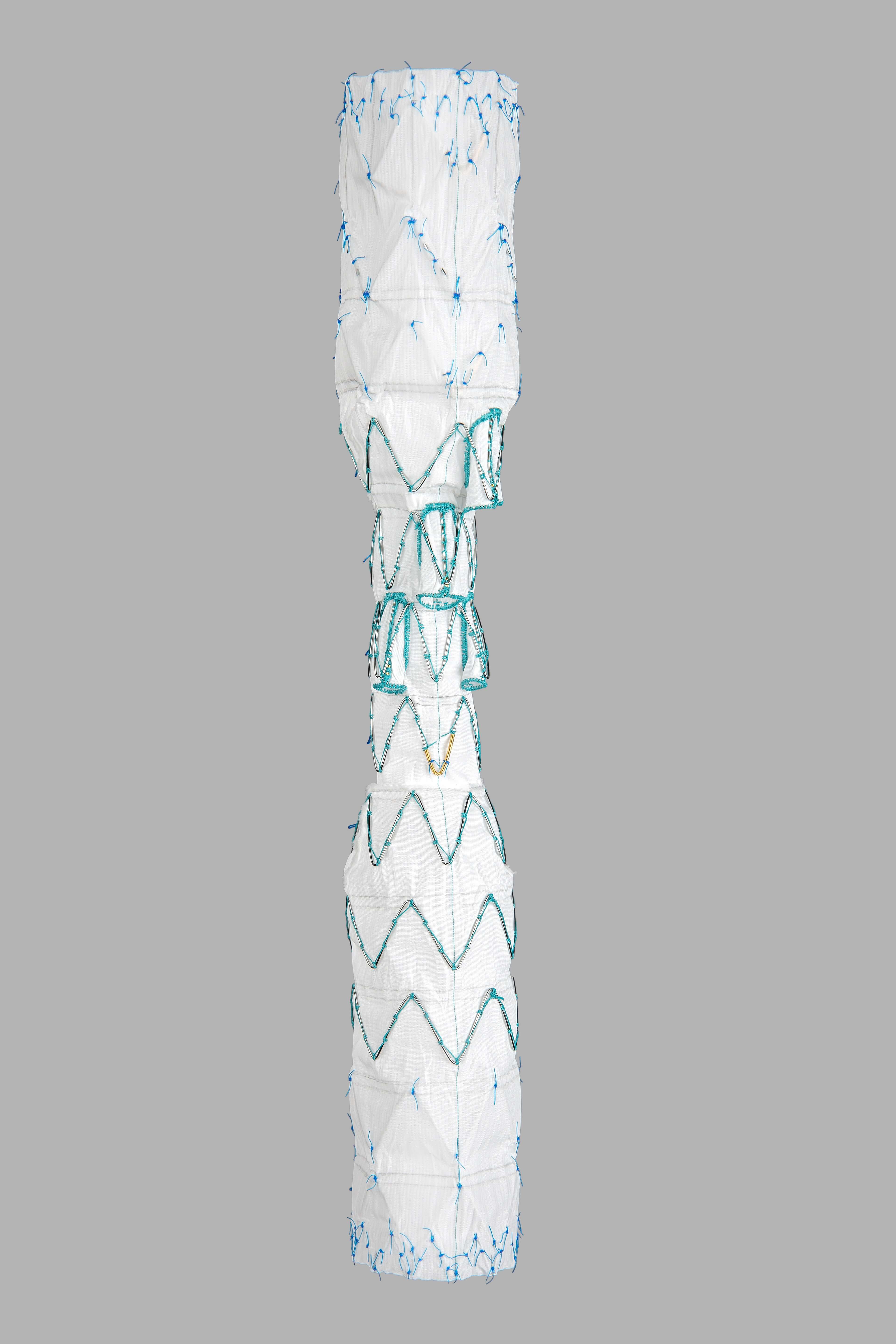 Bloomington, Ind. — Cook Medical’s Zenith® Thoraco+ Endovascular System (Thoraco+) has received Breakthrough Device Designation from the US Food and Drug Administration (FDA). This designation is granted to devices that have the potential to provide more effective treatment or diagnosis…
Bloomington, Ind. — Cook Medical’s Zenith® Thoraco+ Endovascular System (Thoraco+) has received Breakthrough Device Designation from the US Food and Drug Administration (FDA). This designation is granted to devices that have the potential to provide more effective treatment or diagnosis…  Bloomington, Indiana — Cook Medical today announced it signed a letter of intent with CooperCompanies to sell the entirety of Cook’s Reproductive Health business within the MedSurg division. CooperCompanies (NYSE:COO), a publicly held healthcare company, is focused on women’s health and fertility solutions, providing…
Bloomington, Indiana — Cook Medical today announced it signed a letter of intent with CooperCompanies to sell the entirety of Cook’s Reproductive Health business within the MedSurg division. CooperCompanies (NYSE:COO), a publicly held healthcare company, is focused on women’s health and fertility solutions, providing…  Indianapolis, Ind. – Indiana University’s Public Policy Institute (PPI) estimates that Bloomington, Indiana based Cook Medical’s medical device manufacturing facility nearing completion at 38th & Sheridan in Indianapolis will have an estimated economic impact of $25.9 million annually for Marion…
Indianapolis, Ind. – Indiana University’s Public Policy Institute (PPI) estimates that Bloomington, Indiana based Cook Medical’s medical device manufacturing facility nearing completion at 38th & Sheridan in Indianapolis will have an estimated economic impact of $25.9 million annually for Marion… 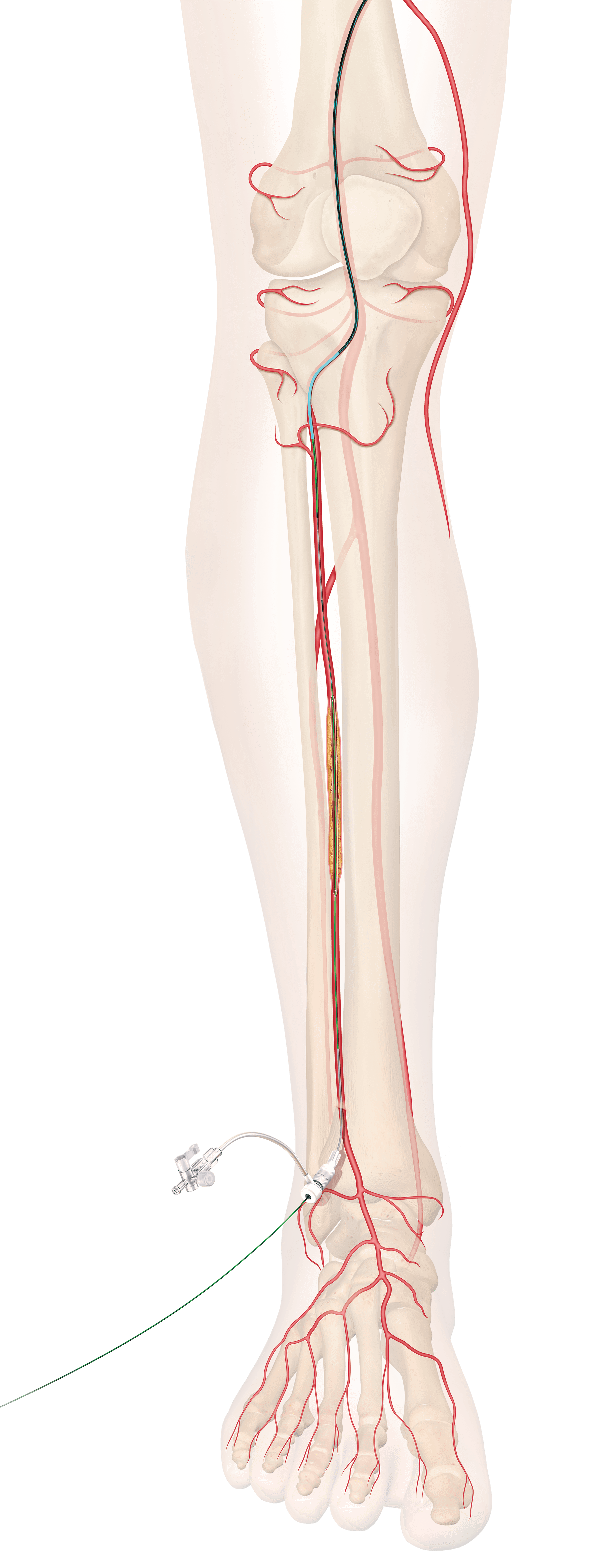 Bloomington, Ind. — Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee (BTK). This new stent is designed to treat patients suffering from chronic limb-threatening…
Bloomington, Ind. — Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee (BTK). This new stent is designed to treat patients suffering from chronic limb-threatening… 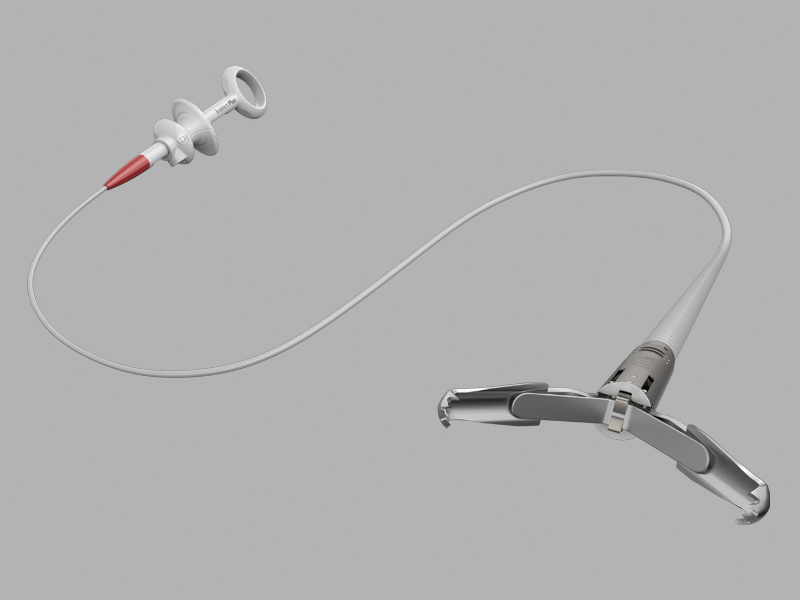 Bloomington, Ind. — Cook Medical’s Instinct Plus® Endoscopic Clipping Device is now commercially available to physicians in the United States. The new clipping device, based on the design of the original Instinct® Endoscopic Clip, features smoother operation and responsive handling1,…
Bloomington, Ind. — Cook Medical’s Instinct Plus® Endoscopic Clipping Device is now commercially available to physicians in the United States. The new clipping device, based on the design of the original Instinct® Endoscopic Clip, features smoother operation and responsive handling1,…  Bloomington, Ind. — Cook Medical has received a three-year contract from Vizient for peripheral vascular devices. The contract allows Cook Medical, which was recently named a finalist for the Vizient 2021 Physician Preference Supplier recognition, to offer negotiated pricing terms…
Bloomington, Ind. — Cook Medical has received a three-year contract from Vizient for peripheral vascular devices. The contract allows Cook Medical, which was recently named a finalist for the Vizient 2021 Physician Preference Supplier recognition, to offer negotiated pricing terms…  Indianapolis, Ind. — What started as a project to address a business challenge and expand manufacturing capacity for Cook Medical has turned into a community collaboration that includes a 100-job manufacturing site and a new grocery store called the Indy Fresh…
Indianapolis, Ind. — What started as a project to address a business challenge and expand manufacturing capacity for Cook Medical has turned into a community collaboration that includes a 100-job manufacturing site and a new grocery store called the Indy Fresh… 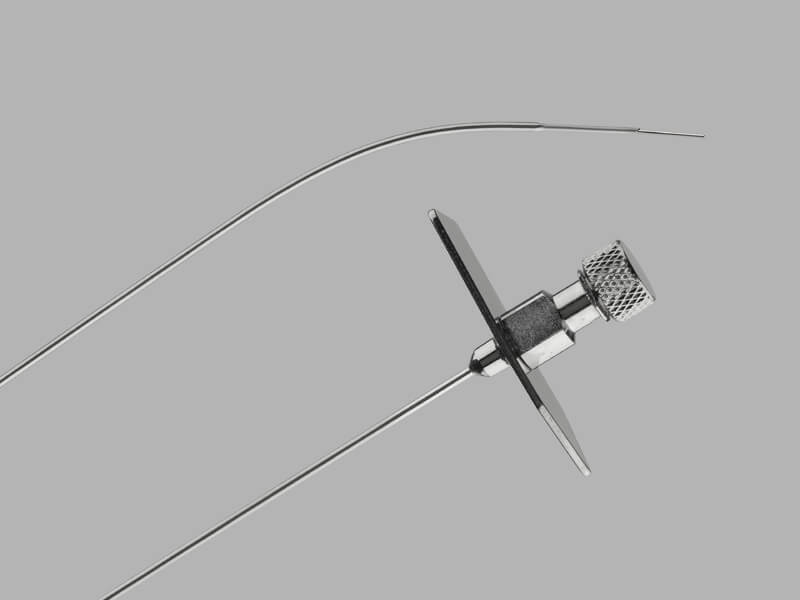 Bloomington, Ind. — On October 8, 2021, Cook Medical issued a global, voluntary recall of the Transseptal Needle and the Transseptal Needle with Catheter. This recall includes all unexpired lots for both of these products. The needles were recalled due…
Bloomington, Ind. — On October 8, 2021, Cook Medical issued a global, voluntary recall of the Transseptal Needle and the Transseptal Needle with Catheter. This recall includes all unexpired lots for both of these products. The needles were recalled due…  Canton, Ill. — Cook Medical is hiring over 100 manufacturing employees and five professional positions at our facility in Canton, Illinois. The expanded manufacturing capabilities will help Cook meet patient and customer needs for medical devices. In addition to providing…
Canton, Ill. — Cook Medical is hiring over 100 manufacturing employees and five professional positions at our facility in Canton, Illinois. The expanded manufacturing capabilities will help Cook meet patient and customer needs for medical devices. In addition to providing… 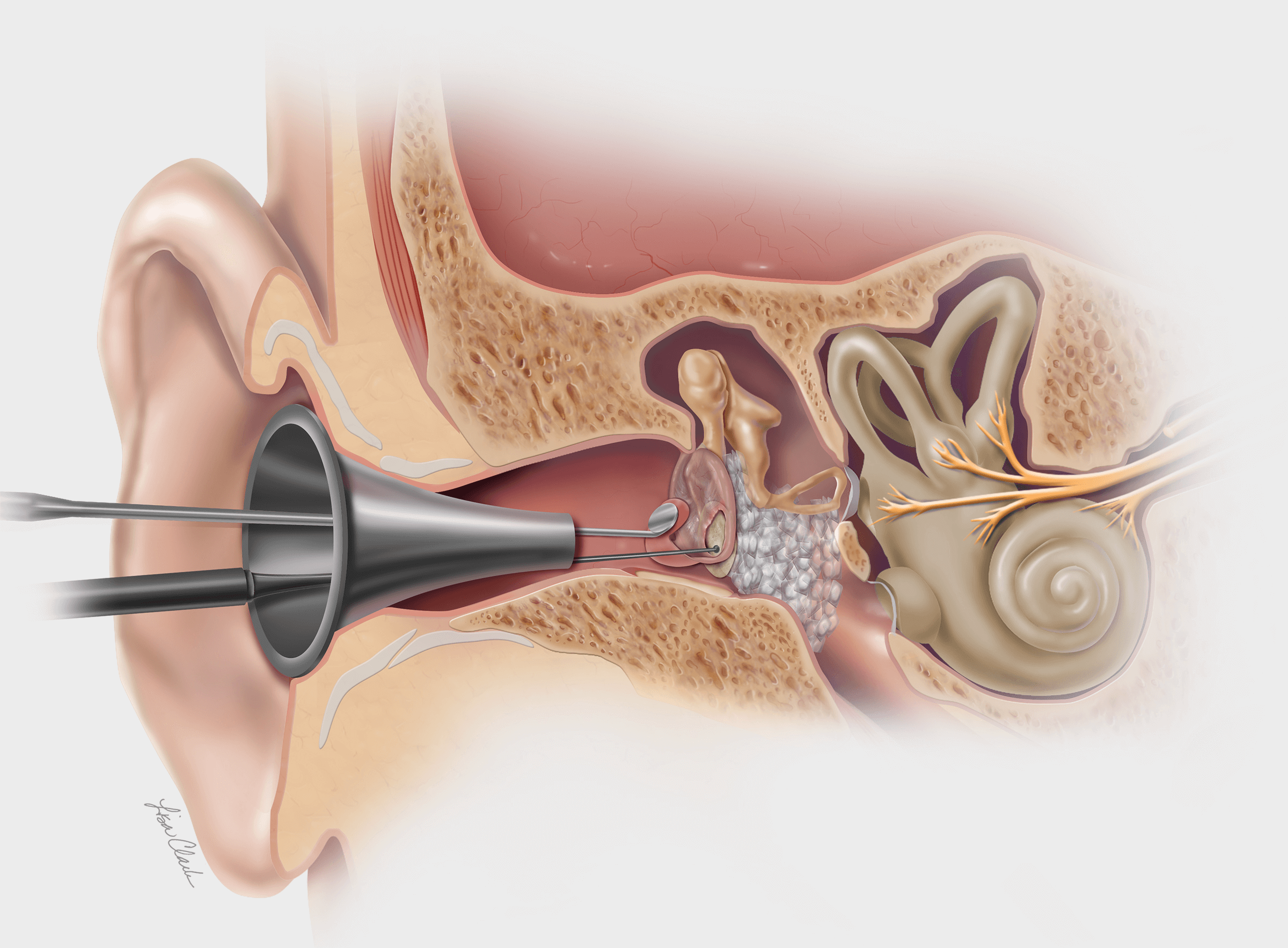 Bloomington, Ind. — Today, Cook Medical announced a new post-market study for the Biodesign Otologic Repair Graft. Otologic repair on perforated eardrums usually requires physicians to harvest patient tissue from elsewhere in the body to repair the membrane. This Cook…
Bloomington, Ind. — Today, Cook Medical announced a new post-market study for the Biodesign Otologic Repair Graft. Otologic repair on perforated eardrums usually requires physicians to harvest patient tissue from elsewhere in the body to repair the membrane. This Cook…  Bloomington, Ind. — A year without Cook Medical’s annual E-Recycle Day led to a record-breaking amount of e-waste collected to be recycled safely and sustainably. Not only was this a record for Cook’s annual Recycle Day, but the event was…
Bloomington, Ind. — A year without Cook Medical’s annual E-Recycle Day led to a record-breaking amount of e-waste collected to be recycled safely and sustainably. Not only was this a record for Cook’s annual Recycle Day, but the event was…  Farah Yousry, a health equity reporter with NPR’s WFYI, Side Effects News and the Indianapolis Recorder, recently shared a story on NPR’s All Things Considered about Cook Medical titled This Neighborhood Badly Needs A Grocery Store. A Medical Device Maker…
Farah Yousry, a health equity reporter with NPR’s WFYI, Side Effects News and the Indianapolis Recorder, recently shared a story on NPR’s All Things Considered about Cook Medical titled This Neighborhood Badly Needs A Grocery Store. A Medical Device Maker…  Demee Koch recently shared an interview with Cook Medical and Cook Group’s president in Medium’s post, Conscious Entrepreneurship: May I introduce Pete Yonkman. Our president, Pete Yonkman, believes companies need to develop deep connections with employees, customers and communities in…
Demee Koch recently shared an interview with Cook Medical and Cook Group’s president in Medium’s post, Conscious Entrepreneurship: May I introduce Pete Yonkman. Our president, Pete Yonkman, believes companies need to develop deep connections with employees, customers and communities in…  Bloomington, Ind. — The Litho 150 laser is now commercially available in the US. The product is the latest offering from Quanta System and is available through Cook Medical distribution. With power of up to 152 W, this ultra-high-power laser…
Bloomington, Ind. — The Litho 150 laser is now commercially available in the US. The product is the latest offering from Quanta System and is available through Cook Medical distribution. With power of up to 152 W, this ultra-high-power laser…  View news coverage of this announcement below. Indianapolis, Ind. — An Indianapolis neighborhood will soon have access to fresh food with a new grocery store, brought to life through a truly unique neighborhood, corporate and non-profit collaboration. The Indy Fresh…
View news coverage of this announcement below. Indianapolis, Ind. — An Indianapolis neighborhood will soon have access to fresh food with a new grocery store, brought to life through a truly unique neighborhood, corporate and non-profit collaboration. The Indy Fresh…  West Lafayette, Ind. — The FDA has reviewed the applications to the Accreditation Scheme for Conformity Assessment (ASCA) Pilot program and has granted laboratory safety accreditation to Cook Research Incorporated (CRI), a part of Cook Medical. CRI is the nonclinical…
West Lafayette, Ind. — The FDA has reviewed the applications to the Accreditation Scheme for Conformity Assessment (ASCA) Pilot program and has granted laboratory safety accreditation to Cook Research Incorporated (CRI), a part of Cook Medical. CRI is the nonclinical…  Cook Medical has a long history working with pioneers and innovators to develop products that are beneficial to both patients and clinicians. For Women’s History Month and beyond, we celebrate our current and past partnerships with women who made a difference. Product name: Rösch-Thurmond Fallopian Tube Catheterization Set…
Cook Medical has a long history working with pioneers and innovators to develop products that are beneficial to both patients and clinicians. For Women’s History Month and beyond, we celebrate our current and past partnerships with women who made a difference. Product name: Rösch-Thurmond Fallopian Tube Catheterization Set…  Bloomington, Ind. — Cook Medical today announced that the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays are commercially available to physicians in the United States and Canada. This product is a new iteration of the Ciaglia Blue Rhino…
Bloomington, Ind. — Cook Medical today announced that the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays are commercially available to physicians in the United States and Canada. This product is a new iteration of the Ciaglia Blue Rhino…  Bloomington, Ind. – The Litho EVO holmium laser is now available through Cook Medical in the United States, Austria, Germany, France, Ireland, Switzerland, and the United Kingdom. The Quanta Litho EVO laser is now available through…
Bloomington, Ind. – The Litho EVO holmium laser is now available through Cook Medical in the United States, Austria, Germany, France, Ireland, Switzerland, and the United Kingdom. The Quanta Litho EVO laser is now available through…  This is a painful time in our country. It’s unfathomable that tragedies such as the deaths of George Floyd, Breonna Taylor, and Ahmaud Arbery continue to happen. Sadly, as people of color know all too well, they do happen every…
This is a painful time in our country. It’s unfathomable that tragedies such as the deaths of George Floyd, Breonna Taylor, and Ahmaud Arbery continue to happen. Sadly, as people of color know all too well, they do happen every…  Bloomington, Ind. — Cook Medical has today officially opened its new state-of-the-art research and development (R&D) Innovation Centre at its plant in Limerick, Ireland. The Innovation Centre together, with the expansion of cleanrooms, packaging, storage and other facilities, represents…
Bloomington, Ind. — Cook Medical has today officially opened its new state-of-the-art research and development (R&D) Innovation Centre at its plant in Limerick, Ireland. The Innovation Centre together, with the expansion of cleanrooms, packaging, storage and other facilities, represents…