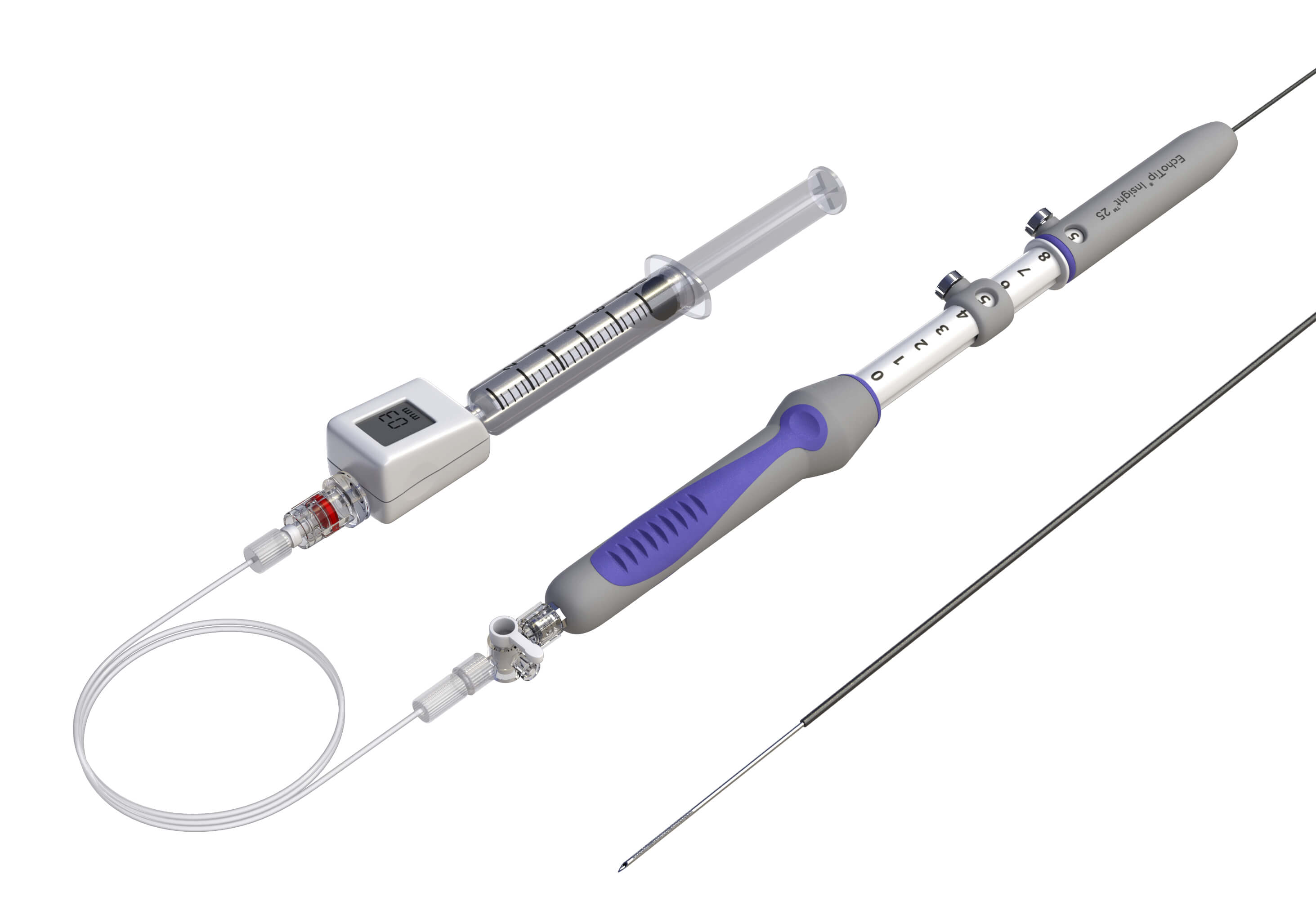
Bloomington, Ind. – The FDA has recently granted Cook Medical a De Novo authorization to market their new device, EchoTip® Insight™ Portosystemic Pressure Gradient Measurement System, in the United States. EchoTip Insight is an endoscopic ultrasound device which allows endoscopists to measure portal hypertension. This authorization is a key step in the preparations to launch the device in the near future.
Using the novel EchoTip Insight measurement system, endoscopists can support a new procedure to perform direct measurement of the portal and hepatic venous pressures under endoscopic ultrasound (EUS) guidance. EchoTip Insight offers an alternative, minimally invasive procedure that can be coupled with a routine esophagogastroduodenoscopy (EGD) examination to help evaluate suspected liver diseases.1 Using EchoTip Insight under EUS guidance, physicians can obtain direct pressure measurements of the portal veins, hepatic veins, and their branches in order to diagnose portal hypertension.2
Conclusions of a recent human pilot study reported the technique of endoscopic ultrasound portal pressure gradient measuring (EUS-PPGM) using a 25-gage needle and compact manometer set-up is feasible.3 This finding may represent a promising advance for procuring valuable information in the management of patients with liver disease.
“We are very pleased to receive this authorization from the FDA to market EchoTip Insight,” said Barry Slowey, president of Cook Medical’s Endoscopy specialty. “It means we’re one step closer to making this device available for physicians and patients. EchoTip Insight has the potential to impact hundreds of thousands of patients living with liver disease and portal hypertension. Once again, Cook Medical is leading the way in innovation in the field of EUS.”
For more information about EchoTip Insight and other endoscopy products, visit CookMedical.com/endoscopy.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: our patients, our employees, and our communities. Find out more at CookMedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
1. Trikudanathan G, Pannala R, Bhutani MS, et al. EUS-guided portal vein interventions. Gastrointest Endosc. 2017;85(5):883–888.
2. Samarasena JB, Chang KJ. Endoscopic ultrasound-guided portal pressure measurement and interventions. Clin Endosc. 2018;51(3):222–228.
3. Huang JY, Samarasena JB, Tsujino T, et al. EUS-guided portal pressure gradient measurement with a simple novel device: a human pilot study. Gastrointest Endosc. 2017;85(5):996–1001.