Bloomington, Ind. — Cook Medical’s Zenith® Thoraco+ Endovascular System (Thoraco+) has received Breakthrough Device Designation from the US Food and Drug Administration (FDA). This designation is granted to devices that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions.
While the product is not commercially available yet, the benefits of the designation include priority review and interactive and timely communication with the FDA during the clinical trial and pre-market review phases in order to help get lifesaving devices to patients more quickly.
The Thoraco+ is the second product from Cook Medical to receive a Breakthrough Device Designation in 2022.
“We are excited to receive an FDA Breakthrough Device Designation for the Thoraco+. This will be a great addition to our portfolio of aortic products so we can offer treatments to a wider variety of patients,” said Mark Breedlove, senior vice president of Cook Medical’s Vascular division.
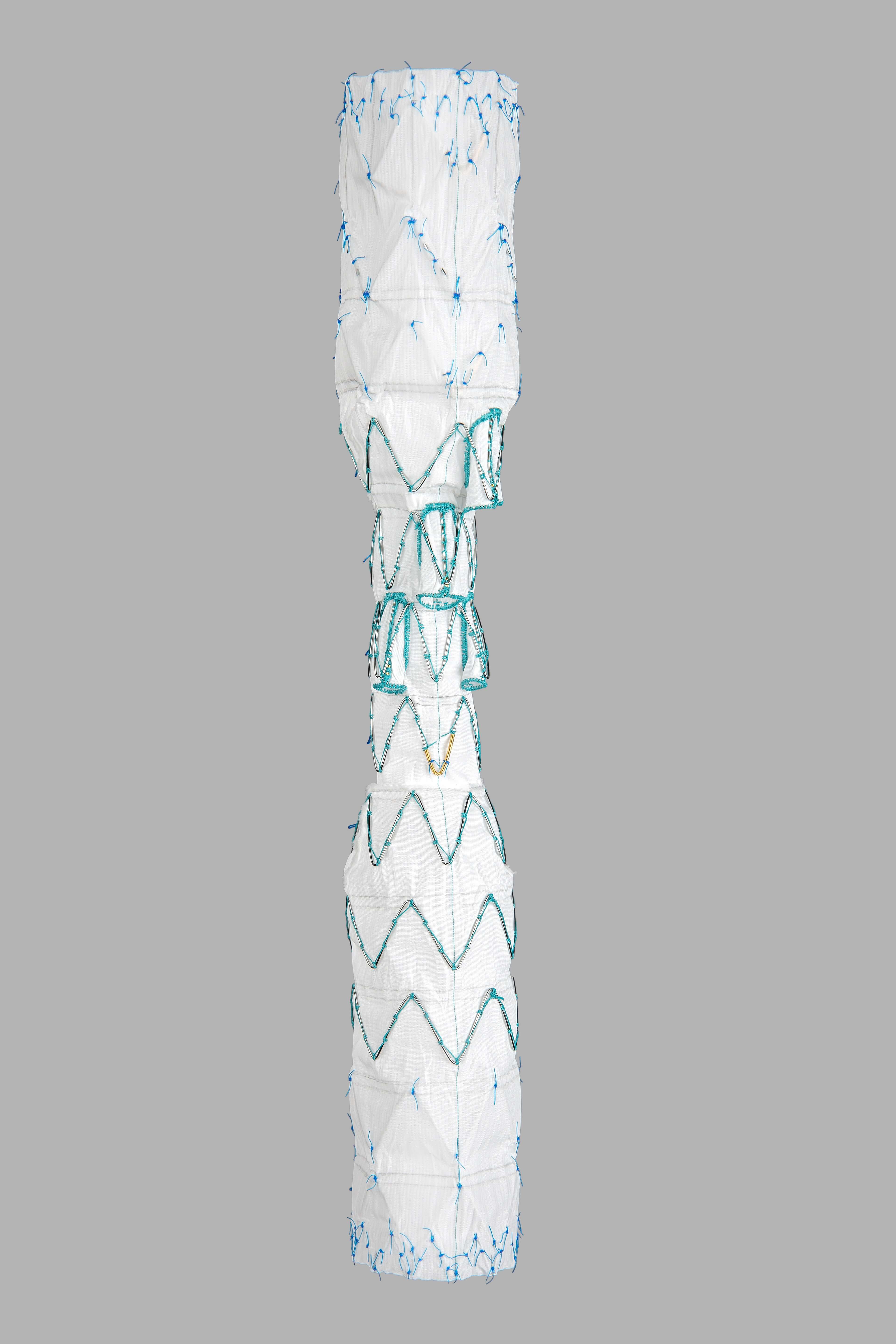
The Thoraco+ is built on the strength of the proven Zenith platform and represents a next generation endovascular graft for the treatment of thoracoabdominal aortic aneurysms. The system is indicated for the endovascular treatment of patients with thoracoabdominal aortic aneurysms (Crawford classification I-IV).
The Thoraco+ is an off-the-shelf device incorporating four side branches for the celiac artery, superior mesenteric artery, left renal artery, and right renal artery. To accommodate varied patient anatomy, the Thoraco+ will be available in a range of diameters and lengths.
To learn more about the Breakthrough Device Designation program, visit the FDA’s website. To learn more about Cook Medical’s aortic intervention products, visit our website.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
News coverage of this announcement
Mass Device—Cook Medical wins FDA breakthrough designation for Thoraco+ endovascular system
Medical Tubing and Extrusion—Cook Medical wins FDA breakthrough designation for Thoraco+ endovascular system
Endovascular Today—Cook Medical’s Zenith Thoraco+ Endovascular System Gains FDA Breakthrough Device Designation
NS Medical Devices—Cook Medical gets FDA breakthrough status for Thoraco+ endovascular system
Medical Device News Magazine—Zenith Thoraco+ Endovascular System Receives FDA Breakthrough Device Designation
Vascular News—Cook Medical receives FDA Breakthrough Device designation for Zenith Thoraco+ endovascular system
Vascular Specialist—Cook Medical receives FDA Breakthrough Device designation for Zenith Thoraco+ endovascular system
Vascular Disease Management—Cook Medical receives FDA Breakthrough Device Designation for Zenith Thoraco+ Endovascular System