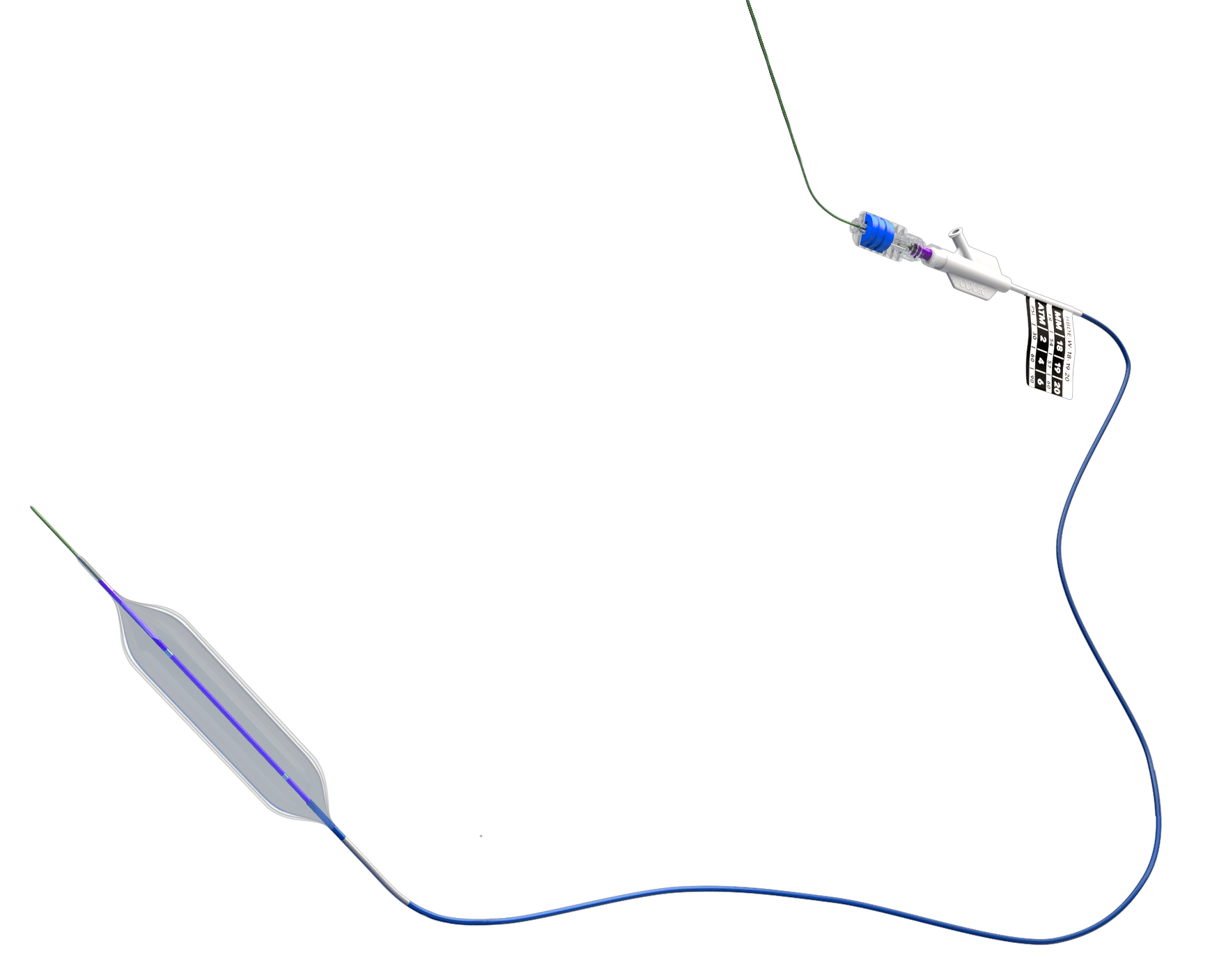
Bloomington, Ind. – Cook Medical announced today that the Hercules® 100 Transnasal Esophageal Balloon is now commercially available to physicians in the US. This product is the first balloon designed specifically for transnasal esophageal procedures. With the Hercules 100, ENT (ear, nose, and throat) physicians have another tool available to help treat esophageal strictures. This is another milestone in Cook’s commitment to supporting less invasive procedures for patients.
“This balloon was specifically designed with ENT physicians and their patients in mind. The balloon is much shorter than what is currently on the market, and it’s tailored to the scope that ENTs commonly use, as well as the patient anatomy they treat,” said Thomas Cherry, director of Cook Medical’s Otolaryngology—Head and Neck Surgery (OHNS) specialty. “Because this procedure can be done in an office, patients can potentially be treated the same day that they are evaluated, rather than having to wait weeks or months to get scheduled into the operating room. This is just one example of how Cook’s OHNS team is innovating minimally invasive options for physicians and patients.”
This new product is a modification of Cook’s existing Hercules 3 Stage Balloon. The Hercules 3 Stage Balloon is designed for gastrointestinal (GI) use, and, by modifying the product, Cook engineers created the Hercules 100 balloon, which is 140 cm shorter than its GI counterpart. Because the Hercules 100 balloon is inserted transnasally, general anesthesia is not required, so the procedure can be done in an office.1 The in-office procedure may have some advantages to similar procedures done transorally in an operating room, such as possible faster recovery times and lower out-of-pocket costs to the patient and the healthcare system.2
In addition to the patient benefits, the product has several features designed to meet the needs of ENTs. For example, it is made with P.E.T. Flex™ material, which combines strength and flexibility for accurate three-stage dilation without conforming to esophageal strictures. The product’s catheter is also compatible with most endoscopes used by ENTs. Additionally, the Hercules 100 can be used with an endoscope camera during the procedure, which allows the physician to see inside the esophagus to make sure the balloon is placed accurately.3
For more information on the Hercules 100 and to see physician training options for this product, visit www.CookMedical.com/Laryngology.
About Cook Medical
Since 1963, Cook Medical has been inventing, manufacturing and delivering a unique portfolio of medical devices to healthcare systems around the world. We work closely with physicians to develop technologies that improve patients’ lives. Because we remain family owned, we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at CookMedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
1 Venkatesan NN, Belafsky PC. Office-based treatment of dysphagia. Oper Tech Otolaryngol Head Neck Surg. 2016;27(2):104–113.
2 Howell RJ, Schopper MA, Giliberto JP, et al. Office-based esophageal dilation in head and neck cancer: safety, feasibility, and cost analysis. Laryngoscope. 2018;128(10):2261–2267.
3 Egan JV, Baron TH, Adler DG, et al. Esophageal dilation. Gastrointest Endosc. 2006;63(6):755–760.