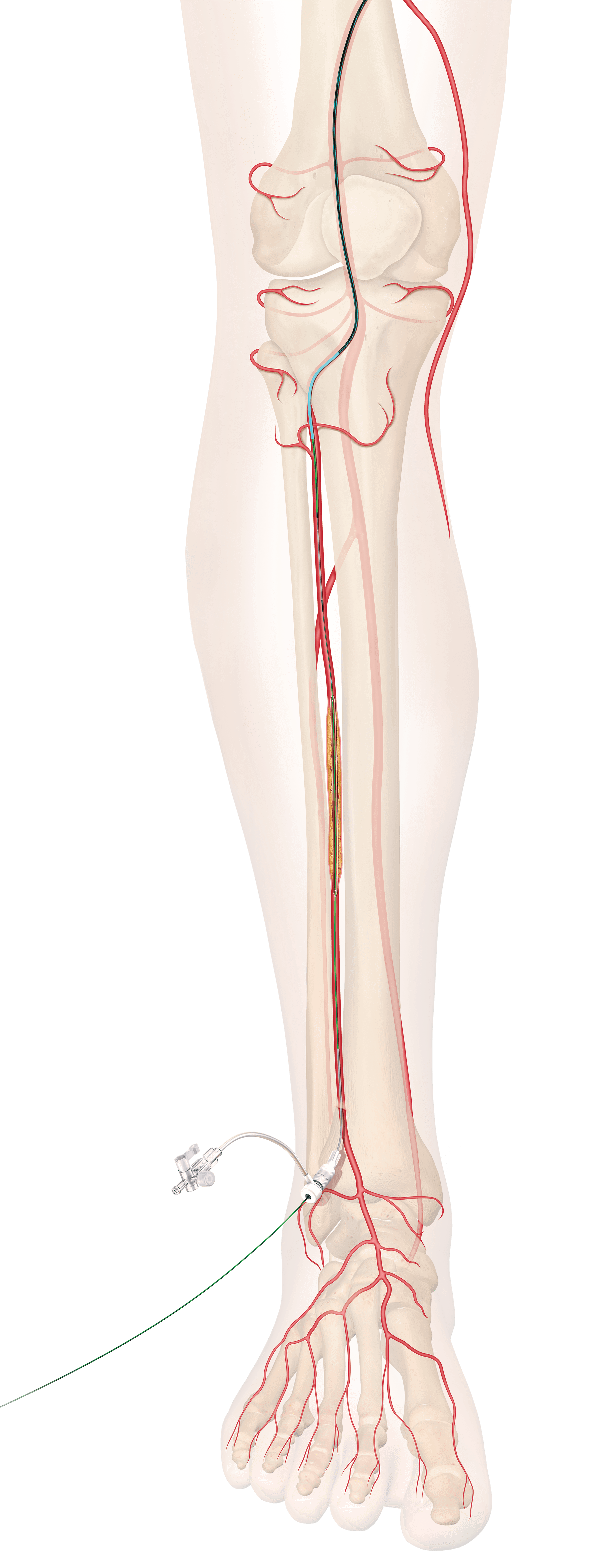
Bloomington, Ind. — Cook Medical has received Breakthrough Device designation from the US Food and Drug Administration (FDA) on a new drug-eluting stent for below the knee (BTK). This new stent is designed to treat patients suffering from chronic limb-threatening ischemia (CLTI).
“CLTI is a debilitating disease of growing prevalence around the globe and this is Cook Medical’s latest innovation within our peripheral artery disease (PAD) program,” said Mark Breedlove, vice president of Cook Medical’s Vascular division. “This new product leverages our deep understanding of stent design and drug elution for lower limb anatomies, and it complements our dedicated portfolio of BTK products for limb preservation. Our goal is to improve the long-term clinical outcomes for CLTI patients.”
The Breakthrough Device designation is granted for devices that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions. While the product is not commercially available yet, the benefits of the designation include priority review and interactive and timely communication with FDA during the clinical trial and premarket review phases in order to help get lifesaving devices to patients more quickly.
To learn more about the FDA’s Breakthrough Devices program, click here. To learn more about Cook Medical’s limb preservation program, click here.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we invent, manufacture and deliver a unique portfolio of medical devices to the healthcare systems of the world. Serving patients is a privilege, and we demand the highest standards of quality, ethics and service. We have remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities.
Find out more at CookMedical.com and for the latest news, follow us on Twitter, Facebook and LinkedIn.
News coverage of this announcement
MD News — Cook Medical Receives FDA Breakthrough Designation for New Drug-eluting Stent
Interventional News– Cook Medical receives FDA breakthrough designation for new drug-eluting stent
Endovascular Today — Cook Medical’s New BTK DES to Treat CLTI Receives FDA Breakthrough Designation
Cath Lab Digest — Cook Medical Receives FDA Breakthrough Designation for New Drug-Eluting Stent
Vascular News — Cook Medical receives FDA breakthrough designation for new drug-eluting stent
Medical Tubing and Extrusion — Cook Medical wins FDA breakthrough designation for new drug-eluting stent
Mass Device — Cook Medical wins FDA breakthrough designation for new drug-eluting stent
Vascular News — Top 10 Stories of 2020: Cook Medical receives FDA breakthrough designation for new drug-eluting stent
Mass Device — Top 10 catheter innovation news stories of 2022: Cook Medical wins FDA breakthrough designation for new drug-eluting stent