Please click the button below and submit the required information to connect with your local Cook representative.
Significant outcomes of the PRESERVE II Clinical Trial
In the video below, Dr. W. Anthony Lee, national principal investigator for the Zenith® Iliac Branch PRESERVE II Clinical Trial, shares his insights on the most significant outcomes from the trial.
Dr. Lee explores the following:
- Highlights of the PRESERVE II trial
- Key factors that influence graft selection
- Physical differences between Cook’s Zenith Iliac Branch device and the GORE EXCLUDER® Iliac Branch Endoprosthesis
Join us as we delve into these important topics with one of the leading experts in the field.
Watch the full interview.
See transcript below.
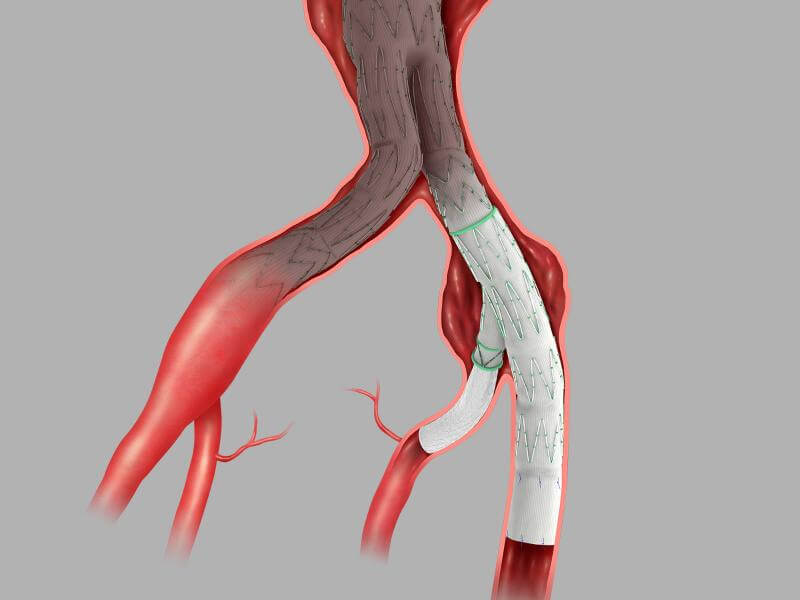
Designed to deliver durable repair.
Designed to deliver durable repair, the Zenith Iliac Branch (ZBIS) performed well in clinical trials. See the pivotal trial data outlined below. These data points provide insights into the long-term performance and clinical benefits of ZBIS.
Pivotal Clinical Trial Data
Primary safety and effectiveness endpoint
6-month freedom from patency-related reintervention1
5-Year Results:
Technical success1
Freedom from buttock claudication1
Number of patients requiring a secondary reintervention on ZBIS side1
Number of occlusions on ZBIS side1
Iliac artery aneurysm enlargement on ZBIS side1
Migration1
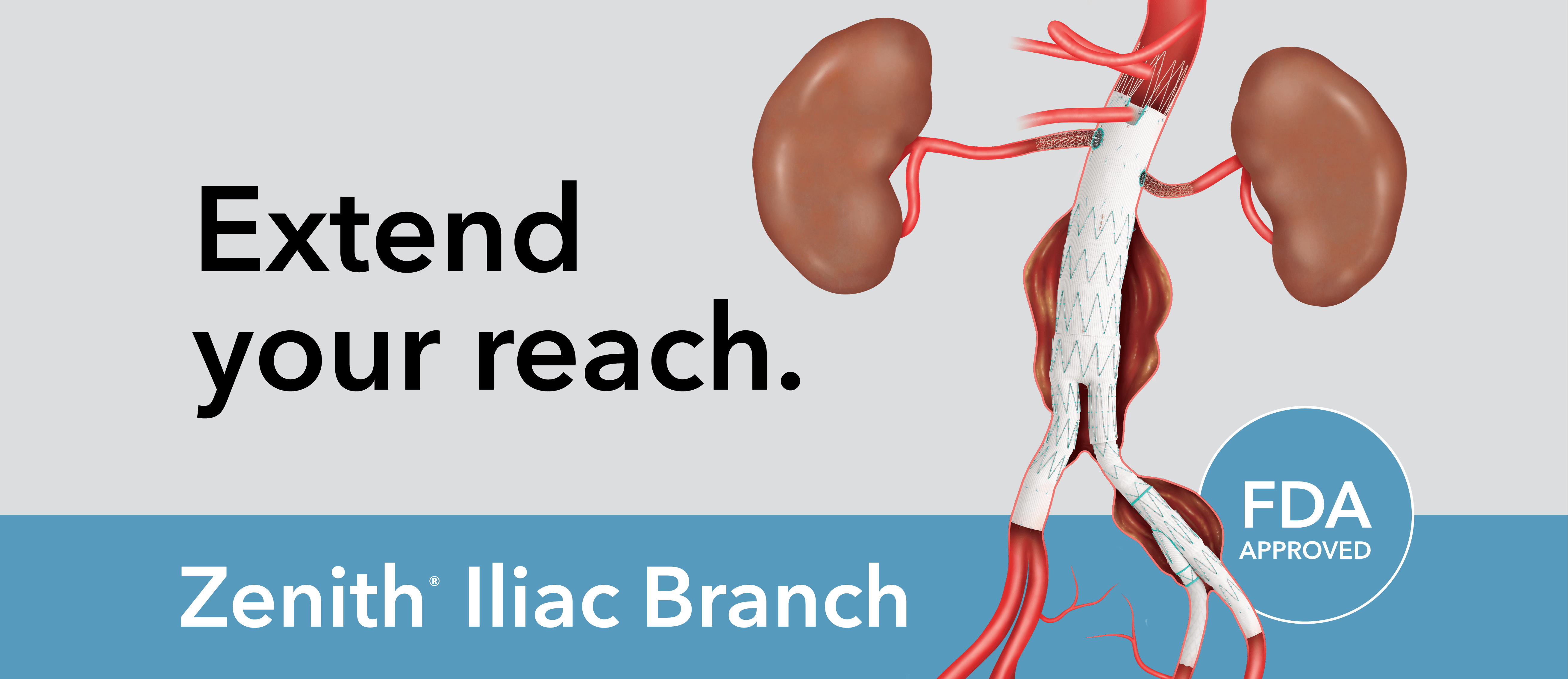
Learn about the Zenith Iliac Branch, watch a deployment video, access planning and sizing sheets, read a product brochure, and more.
Transcript
Title screen (00:00):
Zenith® Iliac Branch
5-year outcomes of the PRESERVE II Clinical Trial
W. Anthony Lee, MD
Principal Investigator, PRESERVE II Clinical Trial
Chief of Vascular Surgery
Christine E. Lynn Heart and Vascular Institute
Boca Raton, Florida
Onscreen disclaimer:
The content, information, opinions, and viewpoints contained in this video are for educational purposes only, and speakers are paid consultants for Cook Medical. Some opinions expressed may represent those of the speaker and are based on their own clinical experience in their practice. This information is not meant or intended to serve as a substitute for a healthcare professional’s clinical training, experience, or judgment. Always refer to the Instructions for Use (IFU) for complete prescribing information including indications for use, warnings, precautions, adverse events, and deployment/use instruction.
Dr. Lee (00:13):
Over the last few years, I’ve had the distinct privilege of being the national PI for the PRESERVE II Clinical Trial.
Slide onscreen (00:21):
What are the most significant outcomes of the PRESERVE II trial?
Dr. Lee (00:26):
Well, the highlights of the PRESERVE II trial can really be summarized by the fact that it not only met but exceeded its primary and secondary endpoints, namely the primary endpoint being patency-related secondary interventions, which was met in 100% of the subjects enrolled.
Onscreen text:
Primary and secondary endpoints include:1
6-month freedom from patency-related interventions was 100%
6-month branch vessel patency was 100%
Dr. Lee (00:48):
I would also like to emphasize that these results have been maintained through the five-year follow-up, which really speaks to the durability of these outcomes.
Slide onscreen (01:01):
With options on the market, what factors into your choice of a Cook device over a Gore device?
Dr. Lee (01:07):
Both devices largely have similar functionalities, but there are substantive physical differences between the two systems that I would like to highlight. First of all, the proximal diameter of the two devices are vastly different. In the Cook IBD device, it’s 12 mm, versus 23 mm of the Gore IBE device. This actually has some relevance in certain anatomies where the proximal portion of the iliac aneurysm can be somewhat narrowed.
The second difference is the Cook IBD device is offered in two main body lengths. In cases where the actual iliac aneurysm is relatively short, this is a very convenient feature, especially in terms of actually deploying this device and accessing from the contralateral side such that the top portion of the endograft does not protrude too much into the aortic aneurysm itself.
Furthermore, the actual iliac branch is also shorter and smaller, and this also impacts the deployment. This once again is an important feature in the context of short common iliac artery aneurysms, where the branch can be positioned extremely closely, right at the opening of the hypergastric artery.
Slide onscreen:
What are the challenges of mixing and matching different company devices?
Dr. Lee (02:46):
But one must keep in mind the outcomes that were obtained and shown in clinical trials, such as the PRESERVE II, really pertain to using the same manufacturer’s devices and staying within the IFU.
Citations
- US Pivotal Clinical Trial Data PMA (P020018/S064). A total of 40 patients were treated with ZBIS and the iCast® covered stent between April 1, 2014, and May 6, 2015. Study patients were enrolled across 18 investigational sites in the United States.
- Dr. W. Anthony Lee was a paid consultant of Cook Medical at the time of recording.
- iCast is a registered trademark of Atrium Medical Corporation.