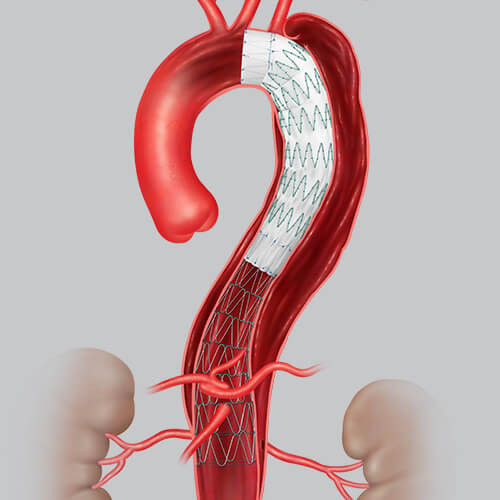
 Following FDA approval of Cook Medical’s Zenith Dissection Endovascular System, physicians are beginning to use the new device to improve the lives of and outcomes for patients who suffer from aortic disease. The system gives physicians a less invasive way to repair Type B dissections of the descending thoracic aorta—a condition that manifests when a tear occurs between the innermost and middle layers of the aorta, allowing blood to flow between the layers and cause them to separate. The condition is life-threatening and can be deadly if left untreated.
Following FDA approval of Cook Medical’s Zenith Dissection Endovascular System, physicians are beginning to use the new device to improve the lives of and outcomes for patients who suffer from aortic disease. The system gives physicians a less invasive way to repair Type B dissections of the descending thoracic aorta—a condition that manifests when a tear occurs between the innermost and middle layers of the aorta, allowing blood to flow between the layers and cause them to separate. The condition is life-threatening and can be deadly if left untreated.
Dr. Eanas Yassa, a vascular surgeon who practices in Grand Rapids, Michigan, first used the device during her fellowship in vascular surgery at Cooper University Hospital in Camden, New Jersey, where the Zenith Dissection Endovascular System was undergoing a trial.
What was your impression of the Zenith Dissection Endovascular System when you used it during your fellowship?
I thought it was a really interesting technology that addressed the problem of not having a minimally invasive, disease specific way to change the natural history of dissection. Typically, when there was a need for emergent surgery, we would place a covered stent over the aorta and then watch and wait to see what happened with the remaining dissections. In many cases, patients would eventually need additional, maximally invasive open surgery to treat the long-term sequela of dissection. That type of operation is highly invasive and involves a high degree of risk for potential complications and a long recovery time.
How does the Zenith Dissection Endovascular System help improve the lives of patients with Type B dissections?
It gives vascular surgeons the ability to shorten the amount of the aorta covered with the stent graft, which lowers the risk for complications associated with the operation1, such as spinal malperfusion and the risk of paralysis. The bare metal stent supports the true lumen, which promotes aortic remodeling2 and makes it less likely that the patient will need more surgery down the line3. Plus, because the System is minimally invasive, recovery time is shorter4.
What’s exciting for you about the System now being available?
It allows me and other vascular surgeons to change the natural history in patients who’ve had a dissection. Before the System was available, dissection patients needed to be constantly monitored and might require major open surgery. Meanwhile, they might have to significantly modify their lifestyle due to having an aortic dissection. Having a minimally invasive tool allows surgeons to intervene earlier, lower the risk for complications, and provide support to the aorta as it attempts to heal itself.
Eanas Yassa, MD, is a paid consultant of Cook Medical.
- Sultan I, Dufendach K, Kilic A, et al. Bare metal stent use in type b aortic dissection may offer positive remodeling for the distal aorta. Ann Thorac Surg. 2018;106(5):1364-1370.
- Melissano G, Bertoglio L, Rinaldi, E, et al. Volume changes in aortic true and false lumen after the “PETTICOAT” procedure for type B aortic dissection. J Vasc Surg. 2012;55(3):641-651.
- Riambau, V, Böckler D, Brunkwall J, et al. Management of descending thoracic aorta diseases clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53(1):4-52.
- Sachs T, Pomposelli F, Hagberg R, et al. Open and endovascular repair of type B aortic dissection in the Nationwide Inpatient Sample. J Vasc Surg. 2010;52(4):860-866.
