A review of the history of the STABLE technique and data from two post hoc comparative analyses comparing these approaches.
BY JONATHAN SOBOCINSKI, MD, PhD; DOMINIQUE FABRE, MD; RICHARD AZZAOUI, MD; AND STÉPHAN HAULON, MD, PhD
Thoracic endovascular aortic repair (TEVAR) is the first-line therapy for acute complicated type B aortic dissection (TBAD).1 The first goal is to treat complications such as malperfusion and/or exclude aortic rupture to save the patient’s life. The principal function of TEVAR is to exclude/cover the main proximal entry tear, thereby redirecting aortic flow exclusively toward the true lumen, and ultimately decreasing the pressure within the false lumen. In a subset of patients with specific anatomic features, early TEVAR might reduce the risk of aneurysmal degeneration during follow-up by promoting early aortic remodeling. Various treatment options have been proposed to reduce early mortality and reduce late aneurysmal degeneration. In this article, we focus on potential benefits of extending TEVAR with a bare-metal stent implanted within the true lumen beyond the thoracoabdominal aortic junction, which is known as the PETTICOAT or STABLE technique.
HISTORY OF THE STABLE TECHNIQUE
Before the advent of TEVAR via stent graft placement, endovascular options were restricted to visceral artery stenting and/or intimal flap fenestration for TBAD.2 Since the first publications in 1999,3,4 TEVAR has evolved to be the best invasive treatment strategy. In our practice, TEVAR for acute TBAD is restricted to complicated cases, defined as aortic rupture (frank or periaortic effusion) and/or organ malperfusion syndrome. However, some authors have suggested consideration of other criteria as well, such as large aortic diameters at onset, refractory pain, and/or persistent hypertension, to define complicated dissections.5
In 2005, Mossop et al published their initial experience combining thoracic endografting with a self-expandable bare stent placed distally,6 which was called the PETTICOAT strategy. This technique has two main goals: (1) to increase the expansion of the true lumen and thus reduce malperfusion, and (2) to promote aortic remodeling. Several authors have reported their experience with this strategy,7,8 and Cook Medical has developed an aortic bare-metal stent specifically for dissection treatment. Cook’s Zenith Dissection endovascular system, comprising a stent graft component and the distal bare stent component specifically for dissection treatment, was evaluated in the STABLE I and II studies.9,10 The STABLE I study assessed an earlier iteration of the device combination (Zenith TX2 stent grafts and stainless steel bare stents) in patients who were treated at up to 90 days from dissection symptom onset and presented with a wide range of indications. The STABLE II pivotal study, conducted later, evaluated the current device system (barbless stent grafts and nitinol bare stents) in patients who presented with only acute, complicated TBAD. Both studies assessed outcomes up to 5 years, but neither compared the results of this combined strategy to endografting alone. We thus conducted two secondary analyses comparing the results from the STABLE cohorts to the results from high-volume European aortic centers, where endografting alone was performed to treat acute complicated TBAD.11,12
AORTIC REMODELING
Aortic remodeling is a combination of true lumen expansion and false lumen thrombosis and shrinkage. It is well described in the literature that TEVAR promotes aortic remodeling at the level of the endograft but has little or no influence on the aorta beyond the diaphragm. There has been scarce literature on the impact of bare stent placement at the level of the thoracoabdominal aorta on aortic remodeling.8,13
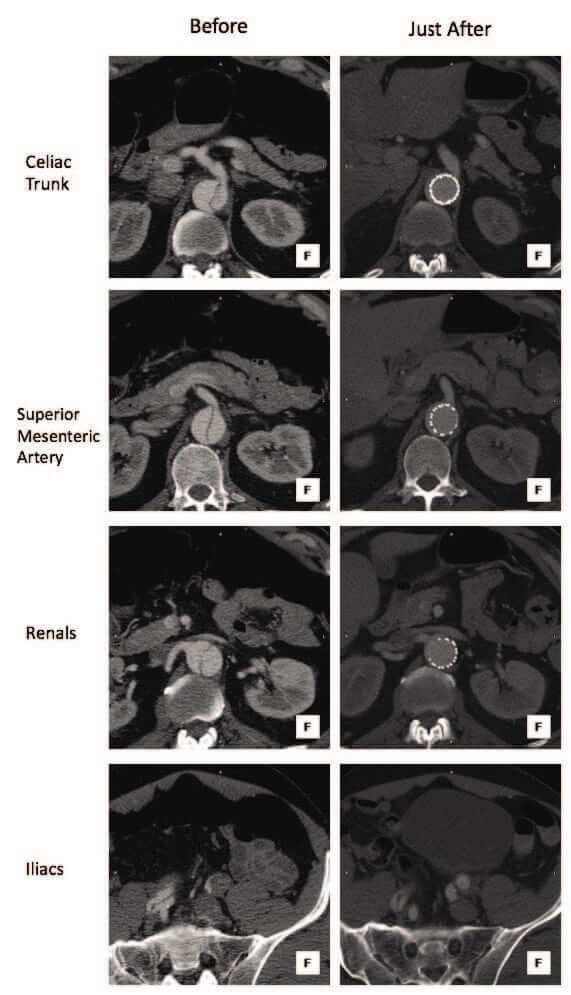
Figure 1. Images of a patient treated for acute complicated TBAD with the STABLE technique. In the left column, slices of the preoperative CTA show collapse of the true lumen along the aorta beyond the thoracoabdominal junction. In the right column, slices of the immediate postoperative CTA exhibit a satisfying opening of the true lumen scaffolding by the bare-metal stent toward the abdominal aorta.
In this context, we conducted a post hoc comparative analysis of two groups of patients surviving 1 year after endovascular treatment of an acute complicated type B dissection, with available CT at baseline and at 12 months: one group was treated with TEVAR alone (n = 45) at three high-volume institutions in Lille, France; Caen, France; and Malmö, Sweden; the other group was treated with the PETTICOAT strategy (n = 39) in the STABLE I study.11 A thorough morphological analysis of the aorta, including changes in aortic volumes, was conducted through 1 year, and details of the initial and secondary procedures were collected.
During the initial procedure, the length of aorta covered by the endograft within the descending thoracic aorta was comparable in both groups (184.0 ± 48.7 mm in TEVAR alone vs 166.6 ± 47.2 mm in STABLE; P = .11). In terms of clinical outcomes at 1 year, the reintervention rates were similar between the two groups (11.1% in TEVAR alone vs 12.8% in STABLE). In terms of aortic remodeling results, while both groups showed significant remodeling in the thoracic aorta (true lumen increase and false lumen decrease in aortic volume), we observed some differences in the abdominal aorta. Only the STABLE group exhibited a statistically significant increase in true lumen volume at the level of the abdominal aorta, most prominently seen on the postoperative as compared to the preprocedure CT scans (P < .001), as well as from postprocedure to 1 year (P = .035), while the changes within the TEVAR alone group were not statistically significant. When compared between the two groups, the overall change in the true lumen volume from preprocedure to 1 year was greater in the STABLE group (16 cm3) than in the TEVAR group (10 cm3) but was not statistically significant (P = .10).
From these results, we hypothesized that this early benefit of true lumen expansion in the abdominal aorta, in relation to the implantation of a bare self-expandable stent in the true lumen, could have an impact on the outcomes of patients presenting with malperfusion at onset (Figure 1). We thus conducted the study described thereafter.
MALPERFUSION
We performed a second post hoc comparative analysis focusing on short-term outcomes of two patient groups treated for acute TBAD with malperfusion (imaging findings and/or clinical signs) diagnosed at onset.12 The first group (n = 41; from Lille, France and Malmö, Sweden) was treated with TEVAR alone, whereas the second group (n = 84; from both the STABLE I and STABLE II studies) was treated with the composite device design.
At presentation, comparable organ system involvement in malperfusion was depicted, and both groups showed similar lengths of dissection and similar locations of the proximal and distal aspects of the dissection. The STABLE patients presented with a higher American Society of Anesthesiologists class, greater prevalence of renal insufficiency, and worse preoperative hypertension and renal function status (according to Society for Vascular Surgery scores) compared with the TEVAR patients. Both groups received a median of one stent graft component (range, 1–2 for TEVAR alone vs 1–3 for STABLE; P = .66). Additional selective stenting of visceral and renal branches was required in 46% of TEVAR patients and 30% of STABLE patients after endograft deployment (P = .08).
The 30-day mortality rate in the STABLE group was half of that in the TEVAR group, but this difference was not statistically significant (8.3% [7/84] vs 17.1% [7/41]; P = .22). Malperfusion-related mortality, defined as deaths caused by bowel/mesenteric ischemia or multiple organ failure, was statistically lower in the STABLE group (2.3% [2/84] vs 12.2% [5/41]; P = .038). The 30-day rates of morbidity such as renal failure requiring dialysis, bowel ischemia, and neurologic events were similar between the groups, as were the 30-day rates of secondary interventions (7.3% for TEVAR alone and 7.1% for STABLE group). Similar to findings from our earlier aortic volume study, the amount of true lumen diameter increase was statistically significantly greater in the STABLE group than in the TEVAR group in the abdominal aorta (P < .001) but not in the thoracic aorta (P = .835).
CONCLUSION
Our volume analysis comparing TEVAR and STABLE showed no statistically significant difference in terms of overall aortic remodeling at 1 year, but the STABLE cohort showed a significant increase of true lumen volume in the abdominal aorta postoperatively. This more prominent true lumen expansion in the distal aorta was also observed at postprocedure in the composite device group in our second study focusing on acute type B dissections in the setting of malperfusion and may have contributed to alleviation of branch vessel malperfusion. In this study, TEVAR + bare-metal stenting showed a twofold reduction in all-cause early mortality, albeit statistically insignificant, and statistically significantly lower 30-day malperfusion-related mortality in patients with acute TBAD with malperfusion compared to TEVAR alone.
Our results suggest that aortic bare-metal stenting in addition to endografting of the proximal descending thoracic aorta should be proposed for patients with malperfusion at onset to improve early survival. Larger cohorts and prospective randomization of patients to both treatment options would be required to confirm these results.
Jonathan Sobocinski, MD, PhD
Aortic Centre
University Hospital of Lille
Lille, France
Disclosures: Proctor and consultant for Cook Medical.
Dominique Fabre, MD
Aortic Centre
Hôpital Marie Lannelongue
Université Paris Sud
Le Plessis Robinson, France
Disclosures: None.
Richard Azzaoui, MD
Aortic Centre
University Hospital of Lille
Lille, France
Disclosures: None.
Stéphan Haulon, MD, PhD
Aortic Centre
Hôpital Marie Lannelongue
Université Paris Sud
Le Plessis Robinson, France
haulon@hotmail.com
Disclosures: Speaker, proctor, and consultant for Cook Medical.
- Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013;61:1661-1678.
- Elefteriades JA, Hammond GL, Gusberg RJ, et al. Fenestration revisited. A safe and effective procedure for descending aortic dissection. Arch Surg. 1990;125:786-790.
- Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539-1545.
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340:1546-1552.
- Tolenaar JL, Froehlich W, Jonker FH, et al. Predicting in-hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation. 2014;130(11 suppl 1):S45-S50.
- Mossop PJ, McLachlan CS, Amukotuwa SA, Nixon IK. Staged endovascular treatment for complicated type B aortic dissection. Nat Clin Pract Cardiovasc Med. 2005;2:316-321.
- Hofferberth SC, Foley PT, Newcomb AE, et al. Combined proximal endografting with distal bare-metal stenting for management of aortic dissection. Ann Thorac Surg. 2012;93:95-102.
- Melissano G, Bertoglio L, Rinaldi E, et al. Volume changes in aortic true and false lumen after the “PETTICOAT” procedure for type B aortic dissection. J Vasc Surg. 2012;55:641-651.
- Lombardi JV, Cambria RP, Nienaber CA, et al; STABLE investigators. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012;55:629-640.e2.
- Lombardi JV, Cambria RP, Nienaber CA, et al; STABLE I Investigators. Five-year results from the Study of Thoracic Aortic Type B Dissection Using Endoluminal Repair (STABLE I) study of endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2019;70:1072-1081.
- Sobocinski J, Lombardi JV, Dias NV, et al. Volume analysis of true and false lumens in acute complicated type B aortic dissections after thoracic endovascular aortic repair with stent grafts alone or with a composite device design. J Vasc Surg. 2016;63:1216-1224.
- Sobocinski J, Dias NV, Hongku K, et al. Thoracic endovascular aortic repair with stent grafts alone or with a composite device design in patients with acute type B aortic dissection in the setting of malperfusion [published online July 4, 2019]. J Vasc Surg.
- Lombardi JV, Cambria RP, Nienaber CA, et al; STABLE investigators. Aortic remodeling after endovascular treatment of complicated type B aortic dissection with the use of a composite device design. J Vasc Surg. 2014;59:1544-1554.
