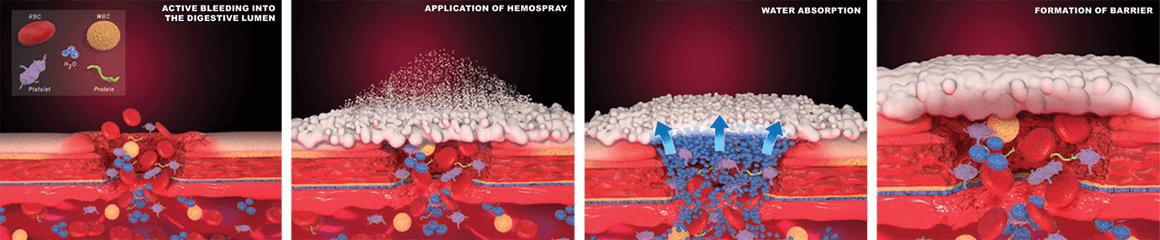
Hemostasis was achieved in 98% of patients treated with Hemospray Hemostatic Endoscopic Hemostat in a new prospective, nonrandomized, multicenter study. Published in the April 2019 issue of Gastrointestinal Endoscopy, the study aimed to evaluate the safety and performance of Hemospray in acute nonvariceal lower GI bleeding. Hemospray, unlike traditional therapies, is a nonthermal, noncontact modality that doesn’t require the precise targeting of other endoscopic devices.
The study followed 50 patients, enrolled across four centers, who presented with active bleeding from a range of different causes in the lower GI tract. Initial hemostasis was achieved in 98% of patients. Comprehensive efficacy and safety follow-ups also reported a 10% rebleed rate within 8-30 days, which is in line with other traditional methods (average rebleed of 12-20% within 7 days1). When evaluating the efficacy of Hemospray, it is also interesting to note that 16 of 38 patients with incident bleeding during colonoscopy were taking anticoagulants or aspirin at the time. Zero therapy-related adverse events were reported.
The authors (Lawrence Hookey, MD; Alan Barkun, MD; Richard Sultanian, MD; and Robert Bailey, MD) concluded: “[Hemospray] is a safe and effective option for patients with lower GI bleeding of varying causes, and in particular, postpolypectomy hemorrhage. The hemostatic powder is effective as monotherapy, part of a combination approach, or as a rescue therapeutic option for the treatment of nonvariceal lower GI bleeding.”1
“The new data further demonstrates the potential for applications of Hemospray to treat patients with GI bleeds, wherever they occur,” said Barry Slowey, president of Cook Medical’s Endoscopy division.
To learn more about the study—titled “Successful hemostasis of active lower GI bleeding using a hemostatic powder as monotherapy, combination therapy, or rescue therapy”—visit the GIE website at https://doi.org/10.1016/j.gie.2018.10.029.
Looking for more information on Hemospray?

Dr. Hookey, Dr. Barkun and Dr. Sultanian are paid consultants for Cook Medical. Dr. Robert Bailey has been a paid consultant of Cook Medical in the past.
1Hookey L, Barkun A, Sultanian R, Bailey, R., 2018,et al. Successful hemostasis of active lower GI bleeding using a hemostatic powder as monotherapy, combination therapy, or rescue therapy, Gastrointest Endosc. 2019;89(4):865-871. doi: https://doi.org/10.1016/j.gie.2018.10.029.
Summary of Clinical Data: Clinical data summary information that was, in part, the basis for granting the de novo can be found on the Cook Medical website at cookmedical.com/HemosprayData