According to Dr. Trerotola, an interventional radiologist at Penn Medicine, the study convincingly showed that “fibers made a huge difference in terms of acute occlusion compared to non-fibered coils”1 in the arterial vasculature.
But this type of study had never been performed in the venous system. Dr. Trerotola and Dr. Sarah White, an interventional radiologist at the Medical College of Wisconsin, along with scientists at Cook Research Inc. (CRI) conducted another first-of-its-kind study: a direct, blinded, head-to-head comparison of fibered vs. non-fibered coils, but this time in a venous setting. In May 2023, the team published their results in theJournal of Vascular and Interventional Radiology in the article “Comparison of Fibered versus Nonfibered Coils for Venous Embolization in an Ovine Model.”
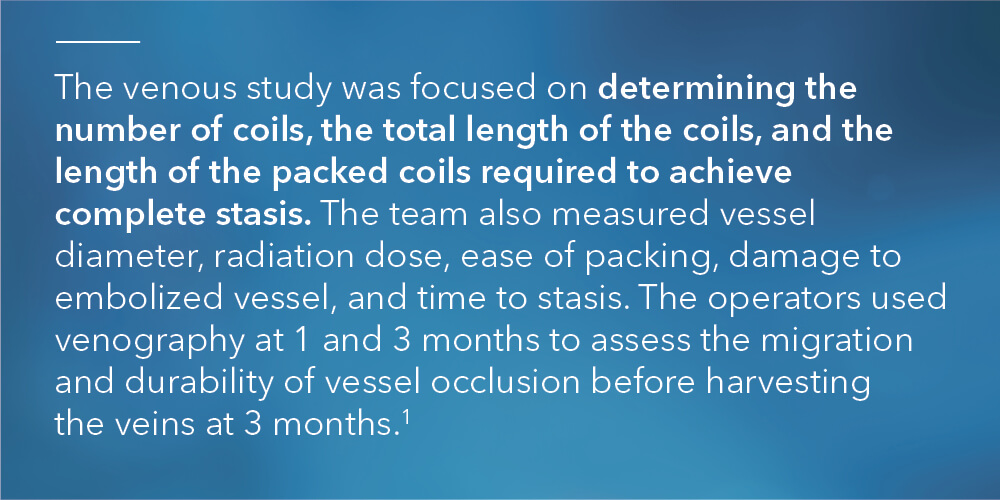
According to Ben Fisher, Cook Medical’s global product manager for embolization coils, “The arterial study was unique because it limited other variables except for fiber in order to focus solely on the performance of fibered vs. non-fibered coils. The venous study design matched the arterial study design in limiting the variables to fiber vs. no fiber. The venous study added some additional primary endpoints, which included testing speed to occlusion and measuring radiation dose.”
Scientists at CRI collaborated on-site with Drs. Trerotola and White to replicate a clinical scenario that had not been studied historically. CRI specializes in clinical research, non-clinical and pre-clinical testing, medical and scientific writing, and regulatory strategy.
CRI operates an in-house histology laboratory that utilizes new techniques to create histology slides and images for pathologists to read. In 2019, the lab developed a state-of-the-art process to create ultra-thin 5 µm (1/5,000th of an inch) slides using a hard plastic resin. While paraffin (wax) histology at this thickness is quite common, ultra-thin hard plastic histology is uncommon.
The plastic resin allows the specimen to include metal, such as from a coil or stent, so researchers can better interpret tissue changes over time. Both thick and thin sectioning are used to create images for studies.
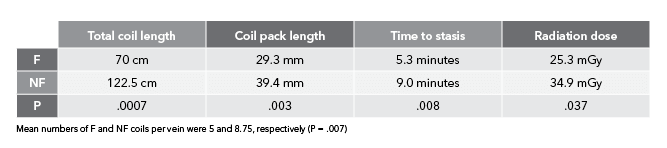
“In the arterial study, we didn’t explicitly look at parameters like time to occlusion and overall radiation dose. We only looked at number of coils needed to achieve occlusion,” Ben said. “We wanted to study those additional parameters this time around so we could draw more conclusions on bare metal vs. fibered coils. This way, when you’re looking at an acute setting, you can say with certainty that you’re going to need fewer coils, it’s going to take less time, and it’s going to use less radiation to achieve the clinical result of occlusion and embolization with fibered coils as compared to non-fibered coils.”
“On the venous side, you’re moving against the blood flow. So, when you inject contrast, it flows back toward the catheter rather than farther downstream into the vasculature, as with arteries,” Ben explained.
“When you place a coil, you’re basically trying to force contrast into the coil pack to see if the veins are going to occlude. It’s difficult in a normal clinical setting to say with 100% certainty whether a vein has occluded or not, just because of the way the contrast flows. So, we had to think of a way around this.”
Drs. Trerotola and White found that solution. They incorporated a second catheter distal to the coil pack. When contrast was injected, it moved in the direction of the blood flow towards and through the coil pack. They were then able to determine if the coil pack was still open or if it had effectively shut off blood flow.
“Normally, the catheter is located proximal to the coil pack. We determined a second catheter was needed distal to the coil pack to inject contrast. This way, the blood flow could carry the contrast toward the coil pack to see if stasis was achieved. If contrast was moving through the coil pack, we added more coils,” Ben explained.
This is a novel technique that could have potential usage in future clinical settings to determine total occlusion.
“When we see in vivo results like this in studies, we hope the same benefits will be applicable to human patients,” said Remco van der Meel, the director of product management for Cook’s Interventional specialty. “We are constantly evaluating our Embolization portfolio, exploring cutting-edge technology, and gathering data to create new technology. Additionally, we want to understand how we could best support our customers in achieving the best possible outcomes for patients. This study helps us make data-based decisions on how to best meet the needs for effective embolization in a cost-efficient manner, while reducing exposure to ionizing radiation for both doctor and patient. These are key elements in today’s healthcare environment.”
References
- Trerotola SO, Pressler GA, Premanandan C. Nylon fibered versus non-fibered embolization coils: comparison in a swine model. J Vasc Interv Radiol. 2019;30(6):949–955.
- White SB, Wissing ER, Van Alstine WG, et al. Comparison of fibered versus nonfibered coils for venous embolization in an ovine model. J Vasc Interv Radiol. 2023;34(5):888–895.