A new prospective, international clinical study1 sponsored by Cook Medical has examined the safety and effectiveness of mechanical and rotational transvenous lead extraction (TLE) of cardiovascular implantable electronic devices (CIED) in real-world patients.
Conducted between October 2018 and January 2020, the Transvenous Lead Removal Using the Cook Evolution® LEAd Extraction SystEm (RELEASE) Post-Market Clinical Study included 230 patients who had 460 leads removed at 10 centers across the US and Europe. Results showed clinical success in 98.7% of procedures with complete procedural success in 96.3% of leads extracted.
There were no isolated extrapericardial SVC injuries and one cardiac injury at the SVC/RA junction. There were no device-related deaths. According to the study’s authors, “These data are in line with the results of a MAUDE database analysis and the PROMET study suggesting that there is a lower risk of isolated SVC injury with the use of rotational TLE tools compared with the use of laser sheaths.”
Rotational lead extraction has been perceived as a slower method of extraction. The RELEASE study set out to record exact start and stop times for each lead removed and the procedure. The results showed the efficiency of Rotational TLE devices.
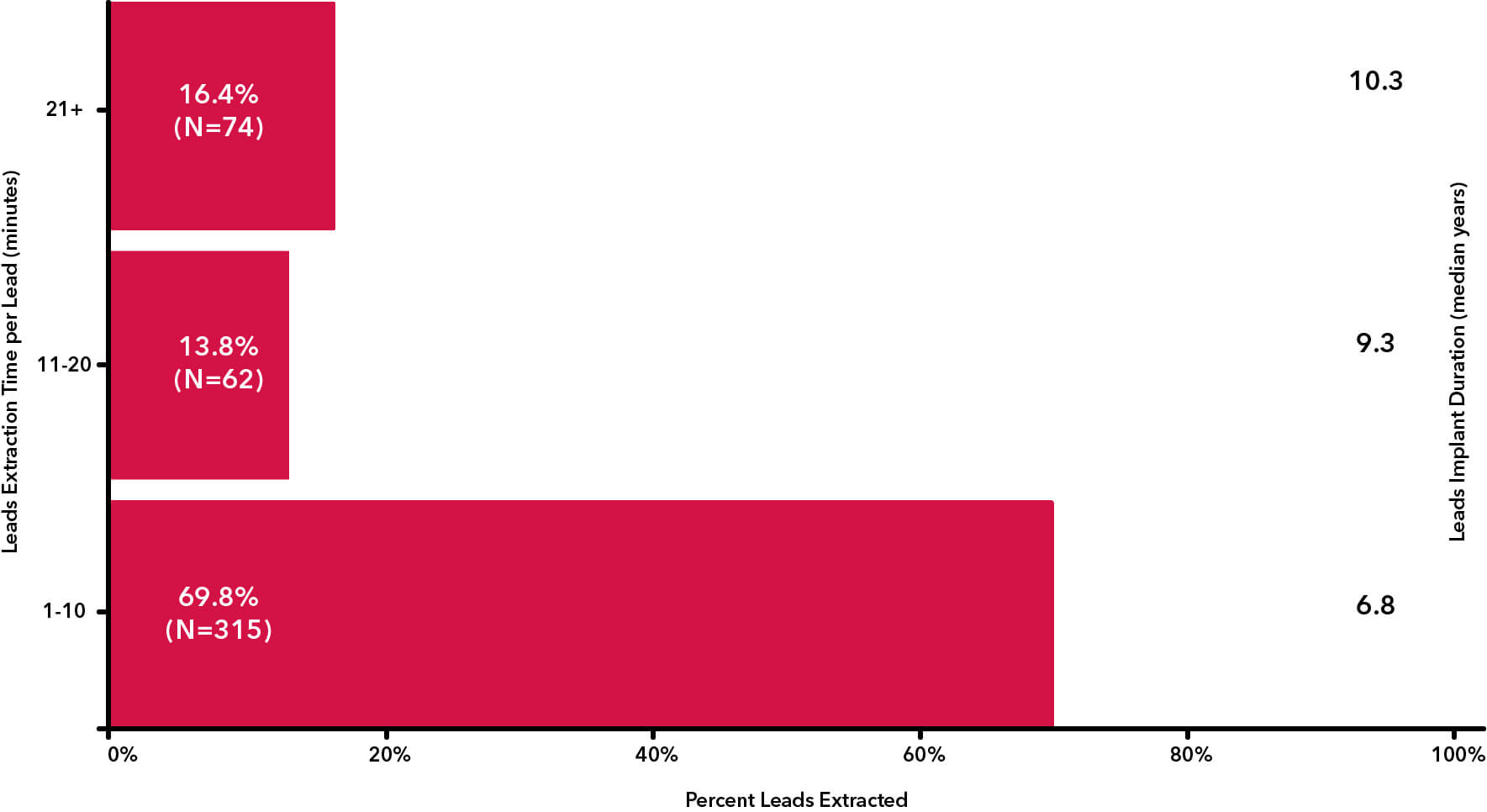
*Graph adapted from Sharma et al. 2021.1
According to the study authors, “RELEASE study results revealed high success rates… despite the long dwell time of targeted leads. Compared with similar TLE studies, this study includes patients with longer lead indwell times and reports similar success rates.” The median indwell time for rotational TLE devices was 7.4 years, and the media extraction time per procedure was 10 minutes compared to the ELECTRa study which had a median indwell time of 5 years and required a median extraction time of 19.0 minutes.
Major device-related complications occurred in 2.6% of procedures, procedure-related major complications were 0.9% within one day. Patient data were source verified, and complications were reviewed and adjudicated by TLE-experienced physicians. According to the physicians, “the RELEASE study may have captured more complications due to the controlled study design (prospective data collection, source data verification by an independent clinical monitoring service and adjudication of all documented events by an independent CEC).”
The RELEASE study evaluated clinical success, procedural efficiency, and complications during patient follow-up on mechanical and rotational devices used to overcome fibrosis and calcification surrounding leads. “The RELEASE study design could serve as a benchmark for future lead extraction studies to better understand the safety of using lead extraction devices.”
“This study represents an advance in TLE trial design, as this study emphasizes the need for comprehensive follow-up and adjudication of all complications by an independent CEC with experience in TLE procedures. Prospective, multicenter, monitored clinical studies will provide a more accurate understanding of the effectiveness and safety of the use of TLE devices.”
Read more about the study here.