Biodesign® Otologic Repair Graft
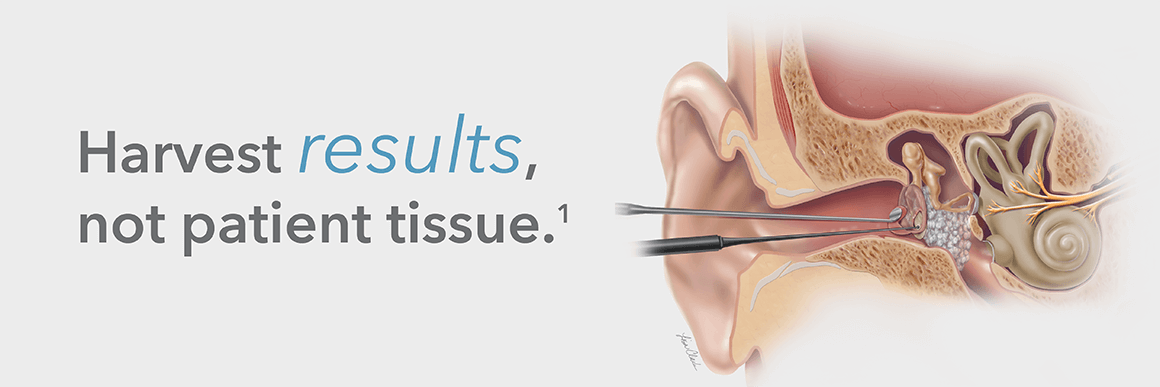
What is the Biodesign Otologic Repair Graft?
The Biodesign Otologic Repair Graft is an off-the-shelf alternative to autologous tissue that provides a
minimally invasive approach to tympanic membrane repair. It eliminates the need for a donor site
and, therefore, no additional scar for the patient.1
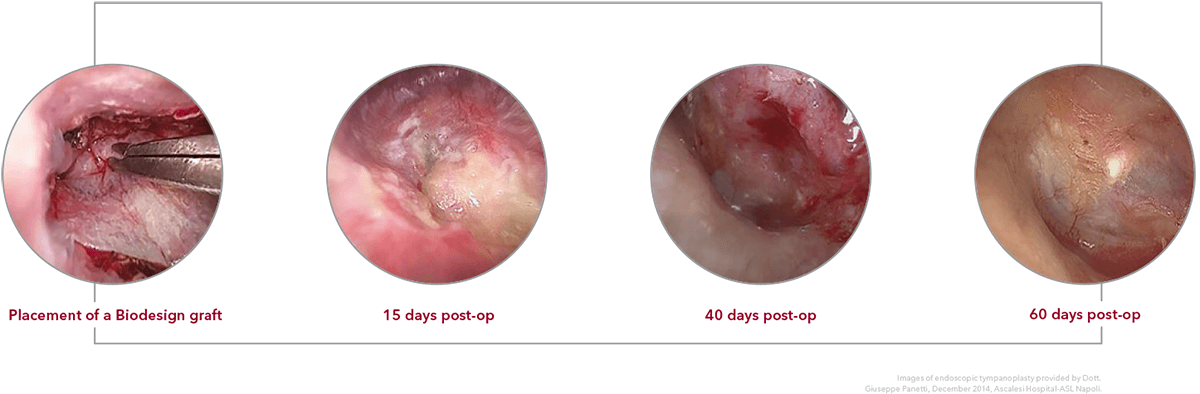
Tissue remodeling
Request a demo
To be connected with your local Cook Medical representative, please provide the information below. Please see our Privacy Statement for data protection notices relating to our collection and use of your data.
Features and benefits
The Biodeisgn Otologic Repair Graft enables a minimally invasive approach to ear surgery with no donor site required and, therefore, no additional scar for the patient.
Reliable closure
Biodesign material remodels into natural host tissue with an overall success of 91% across published literature1-9 and no statistically significant difference in audiometric results when compared to temporalis fascia.1, 10

Excellent handling
Biodesign material is easy to manipulate, allowing for improved surgical precision during graft placement.1

Time saving
The Biodesign Otologic Repair Graft reduces the need to harvest autologous tissue, significantly decreasing intraoperative time.1