Cook Medical’s Zilver Vena Venous Self-Expanding Stent has shown high rates of sustained patency as well as freedom from clinically driven intervention over 3 years, according to recently published data in the Journal of Vascular and Interventional Radiology (JVIR). This self-expanding venous stent is used in the treatment of symptomatic iliofemoral venous outflow obstruction.
The prospective, multicenter, nonrandomized, single-arm study demonstrates sustained high patency rates as assessed by Kaplan–Meier (KM) estimates, as well as significant clinical improvement in scoring over the study’s duration, compared to the baseline.1 The study included 243 patients with obstruction originating from lesions after the removal of acute deep vein thrombosis (aDVT), from postthrombotic syndrome (PTS), and from nonthrombotic iliac vein lesions (NIVL), representing a real-world patient population.1
Terminology explained
In venous stenting, ensuring long-term effectiveness is a key component for improving patient outcomes. Patency, or the openness of a blood vessel after treatment, is a measure of effectiveness, as it indicates whether the stent is allowing proper blood flow without blockage. The VIVO study reports high rates of sustained patency over 3 years.1
The study also examined various patient subgroups, including those with conditions such as aDVT, PTS, and NIVL, in order to understand how different populations respond to the treatment. aDVT is a blood clot that forms in a deep vein, which can lead to immediate symptoms such as pain and swelling, where PTS may occur as a long-term complication following a blood clot such as aDVT; it can lead to chronic leg pain, swelling, and skin changes. NIVL refers to a condition in which blood flow in the iliac veins is obstructed without the presence of a clot, often due to external compression. Understanding these subgroups helps tailor medical solutions to diverse patient needs.
Key study findings
- Sustained overall patency rate: The 3-year patency rate by ultrasound was 90.3±2.2%1
- Sustained high patency across all patient subgroups-KM estimates for patency by ultrasound at 3 years were as follows:
- 84±5.8% for the aDVT group1
- 86.1±3.9% for the PTS group1
- 100% for the NIVL group1
- Sustained safety and performance: The rate of freedom from clinically driven reintervention for the patient cohort was 92.6% at 3 years.1
- Freedom from stent fracture: No fractures were observed throughout the study duration, demonstrating stent durability.1

*Patency by ultrasound (flow or no flow)
Improvements in clinical scoring
Effectiveness was further demonstrated by improvements in clinical scoring from the baseline, including the venous clinical severity score (VCSS), the chronic venous disease quality of life questionnaire (CIVIQ-20), the venous disability score (VDS), and the CEAP-C classification (which measures clinical, etiological, anatomical, and pathophysiological categories).1
The CIVIQ-20 measures a patient’s quality of life as indicated by pain, physical functioning, social limitations, and emotional well-being, while the VDS assesses the functional disability caused by the venous disorder. By evaluating a patient’s ability to perform daily activities, the VDS allows clinicians to track disease progression and treatment effectiveness. The VCSS is commonly used to assess the severity of venous disease based on clinical signs such as edema, skin changes, and ulceration.
These clinical scoring systems not only demonstrate the efficacy of treatments but also help improve patient outcomes by providing standardized, measurable ways to assess venous disease severity and its impact on quality of life.
Patients with aDVT
- Patients with aDVT saw a significant 4.8-point decrease in their symptoms at 3 years according to the VCSS.1 In addition, their CIVIQ-20 scores improved by 26.2 points over 3 years, demonstrating improvement in patient symptoms as compared to baseline1
- The freedom from clinically driven reinterventoon rate was 92.1 ± 4.1 for the aDVT group at 3 years.1
Patients with PTS
- For PTS patients, the VCSS decreased by 3.5 points, indicating marked alleviation of symptoms as compared to baseline, where the CIVIQ-20 scores improved by 20.8 ± 20.4 points over 3 years.1
- The freedom from clinically driven reintervention rate was 87.1 ± 3.9% for the PTS group at 3 years.1
Patients with NIVL
- Finally, NIVL patients experienced a 4.4-point decrease in the VCSS and a 17.9-point improvement in their CIVIQ-20 scores at 3 years.1
- The freedom from clinically driven reintervention rate was 100% for the NIVL group at 3 years.1

Furthermore, after stent placement, patients reported fewer symptoms over time according to CEAP-C classification, and the correlation between time points through 3 years and CEAP-C classification was highly significant (p < 0.0001; nonzero correlation, Cochran–Mantel–Haenszel test).1
Conclusion
The study’s key findings highlight the stent’s effectiveness and durability, demonstrated by high patency, freedom from clinically driven reintervention, freedom from stent fracture, and improvement in venous clinical symptoms across a diverse patient group.1 These results indicate the stent’s effectiveness in maintaining vessel patency, which reduces the need for additional interventions and improves patients’ overall quality of life.1
Implications: The Zilver Vena Venous Self-Expanding Stent is effective for treating symptomatic iliofemoral venous outflow obstruction, yielding reliable clinical improvements and high patency rates.1
Learn more about Zilver Vena.
- Comerota AJ, Gagne P, Brown JA, et al.; VIVO clinical study investigators. Final 3-year study outcomes from the evaluation of the Zilver Vena venous stent for the treatment of symptomatic iliofemoral venous outflow obstruction (the VIVO clinical study). J Vasc Interv Radiol. 2024;35(6):834–845. https://doi.org/10.1016/j.jvir.2024.02.025
Please see product risk information in the IFU at cookmedical.com.


While the treatment of venous obstruction has seen significant advancements in recent years, there continues to be a lack of robust evidence or standardized best practices for venous specialists to follow. This has been particularly challenging for those managing lower extremity venous outflow obstruction in their patients.
To help establish protocols to enhance clinical outcomes and guide future research, Cook Medical brought together a coalition of physicians to participate in a Delphi consensus study. The Delphi technique leverages the collective opinion of expert panel members through a systematic, multi-round process. This method, which is particularly effective in areas with sparse research, provides a structured framework in which experts can reach an agreement.
The results were published online in the October 2023 edition of the European Journal of Vascular and Endovascular Surgery.
In this study, the Delphi process was executed over two rounds. Initially, six expert physicians from the US and Europe formulated 40 statements covering various aspects of venous obstruction management. In the second round, the statements were expanded to 80 and reviewed by a broader panel of 24 physicians. The statements were then distributed to a carefully chosen group of venous experts who met specific qualifying criteria.
Statements were rated on a 9-point Likert scale. Consensus was achieved when at least 70% of respondents rated a statement between 7 and 9 (agreement) or 1 and 3 (disagreement). Feedback from the first round was used to refine the statements for greater clarity in the second round.
Results of the consensus study
In the first round, 75 out of 110 experts (68%) responded, reaching consensus on 32 of the 40 statements (80%). In the second round, 91 out of 121 experts (75%) responded, with consensus achieved on 50 of the 80 statements (62.5%).
Key areas of consensus
- Imaging: 2 out of 3 statements (67%)
- Symptoms and baseline measures: 12 out of 24 statements (50%)
- Differential diagnosis: 2 out of 8 statements (25%)
- Treatment algorithm: 10 out of 17 statements (59%)
- Indications for stenting: 10 out of 10 statements (100%)
- Inflow and outflow assessment: 2 out of 2 statements (100%)
- Procedural outcomes: 2 out of 2 statements (100%)
- Post-procedure therapies and stent surveillance: 5 out of 7 statements (71%)
- Clinical success factors: 5 out of 7 statements (71%)
The consensus study highlighted considerable agreement on optimal management practices for lower extremity venous outflow obstruction. However, it also underscored areas where consensus was lacking, notably in the treatment algorithm section.
Areas requiring further research
- Treatment algorithms
- Procedural interventions
- Approach to treating deep versus superficial venous disease
- Post-intervention anticoagulation strategies
For physicians, the Delphi consensus study helps provide valuable insights into current expert recommendations and identifies areas where further evidence is needed. By adhering to the consensus guidelines and focusing on the highlighted areas for additional research, the medical community can work toward more effective and standardized treatments for venous obstructive disease.
“In the absence of robust clinical data, practicing physicians have been left to draw parallels to venous interventions from their prior arterial interventional experience,” said Dr. Lawrence “Rusty” Hofmann, professor of radiology at Stanford Medicine and senior author of the study.
“Unfortunately, from a fluid dynamics perspective, the venous system is a capacitance system, whereas the arterial system is a resistance system. So, there is not a one-to-one translation of patient selection nor techniques. Therefore, the consolidation of the world’s experts into a consensus document is an important step forward in guiding treating physicians on how to achieve the best outcomes for patients,” he said. “Through studies like this, we can better understand the complexities of venous outflow obstruction and improve patient outcomes by developing evidence-based best practices.”
According to Bruce Fleck, clinical programs manager for Cook Medical, this consensus study marks a significant step toward establishing best practices for treating venous outflow obstruction. “Achieving considerable agreement among a global group of experts affirmed much of what we thought were best practices for this patient population,” he said. “The areas lacking consensus highlight critical topics for future research and investigation.”
About the authors
The study was conducted by a diverse team of six authors, three from the US and three from the EU, representing the specialties of vascular surgery, interventional radiology, and interventional cardiology. Their combined expertise and collaborative effort were crucial in achieving the study’s goals.
Coming this fall: Cook Medical is sponsoring an online CME course in partnership with HMP beginning Aug. 1, 2024. Sign up here.
- Black SA, Gohel M, de Graaf R, et al. Management of lower extremity venous outflow obstruction: results of an international Delphi consensus. Eur J Vasc Endovasc Surg. 2024;67(2):341–350. E-pub 2023 Oct 4. doi:https://doi.org/10.1016/j.ejvs.2023.09.044
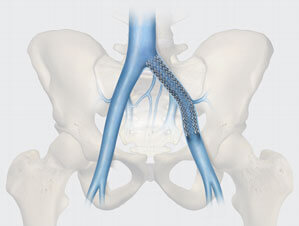 Study suggests effective treatment of symptomatic iliofemoral venous outflow obstruction1
Study suggests effective treatment of symptomatic iliofemoral venous outflow obstruction1
The study, “Twelve-month end point results from the evaluation of the Zilver Vena venous stent in the treatment of symptomatic iliofemoral venous outflow obstruction (VIVO clinical study),” has shown that Zilver Vena is a safe and effective treatment option for patients diagnosed with symptomatic iliofemoral venous outflow obstruction.1
The VIVO clinical trial was conducted between December 2013 and October 2016 and was published in the Journal of Vascular Surgery Venous and Lymphatic Disorders. The multicenter study enrolled 243 patients in the United States and Taiwan with symptomatic venous outflow obstruction of one iliofemoral venous segment. All participants received the Zilver Vena stent.1
“One in three Americans over the age of 45 has some kind vein disease,”2 according to the American Venous Forum. With chronic venous disease affecting 25 million adults in the United States,3 accessible treatment is crucial to maintaining quality of life for these patients, including those experiencing iliofemoral venous outflow obstruction.
Chronic venous outflow obstruction commonly refers to long-term stenotic and occlusive disease of the central veins or to iliofemoral outflow obstruction. Severe and chronic obstruction of the iliac veins can result in life-altering symptoms including chronic pain, while treatment options like anticoagulant therapy are not one-size-fits-all approaches to treating venous conditions.
The VIVO clinical study results showed that “the 30-day freedom from major adverse events rate was 96.7%, greater than the literature-defined performance goal of 87%.”1 Similarly, “the 12-month primary quantitative patency rate was 89.9%, greater than the literature-defined performance goal of 76%.”1
“The primary safety end point was 30-day freedom from major adverse events,”1 and “the primary effectiveness end point was the 12-month rate of primary quantitative patency by venography as determined by the core laboratory.”1
The study also found that the Zilver Vena stent led to clinical symptom improvement compared with the baseline. The change in the Venous Clinical Severity Score (VCSS) from baseline was -3.0 at one month and and -4.2 at 12 months, respectively.1
The Zilver Vena stent has been approved by the US Food and Drug Administration (FDA) since October 9, 2020.
- Hofmann L, Gagne P, Brown JA, et al.; VIVO study investigators. Twelve-month end point results from the evaluation of the Zilver Vena venous stent in the treatment of symptomatic iliofemoral venous outflow obstruction (VIVO clinical study). J Vasc Surg Venous Lymphat Disord. 2023;11(3):532–541.e4. doi: 10.1016/j.jvsv.2022.12.066.
- What is vein disease and lymphedema? American Venous Forum Web site. https://www.venousforum.org/patients/what-is-vein-disease. Accessed on June 15, 2023.
- Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130(4):333–346.
Please see product risk information in the IFU at cookmedical.com.
An interview with Dr. Eric Secemsky

Eric A. Secemsky, MD, MSc, RPVI, FACC, FAHA, FSCAI, FSVM
Director of Vascular Intervention, Beth Israel Deaconess Medical Center Section Head, Interventional Cardiology and Vascular Research, Smith Center for Outcomes Research
Assistant Professor of Medicine, Harvard Medical School
On July 11, 2023, the FDA reversed a decision outlined in a controversial 2019 letter on the potential increase in all-cause mortality when using paclitaxel-coated devices compared with non-drug-coated devices to treat peripheral arterial disease (PAD) in the femoropopliteal artery.
In the updated letter, the FDA stated “the data does not support an excess mortality risk for paclitaxel-coated devices.” Physicians around the world celebrated the FDA’s conclusion that these devices are safe and effective for patients suffering from PAD.
Dr. Eric Secemsky, a primary investigator on SAFE-PAD (Safety Assessment of Femoropopliteal Endovascular Treatment with Paclitaxel-Coated Devices),1 played an important role in helping bring these lifesaving devices back to the shelves.
We recently spoke to Dr. Secemsky about the fallout and subsequent turnabout regarding this important technology.
How will the FDA’s updated letter positively impact patients?
Many of us practicing in the vascular space, particularly in the US, felt confident that there were no safety concerns with these devices. Obviously, any suspicion leads to caution among the general group of operators because our patients are always the priority. The FDA is charged with protecting the safety of our patients in the US from a regulatory standpoint, and they now agree that there is no clear evidence of safety issues with paclitaxel-coated devices. That is a stamp of approval that was needed for us to move back to our routine practice and deliver the best care for our patients.
A notable impact of the FDA’s decision is that it allows other non-US regulatory bodies to consider similar endorsements for more routine use of these devices. Hopefully now every patient can benefit from this technology no matter where they live.
What was your role in researching paclitaxel and its long-term impact?
I got involved very early after the Katsanos meta-analysis came out. Our research center in Boston (Smith Center for Outcomes Research) had put out two papers1,2 using real-world evidence from a claims registry that demonstrated no reproducibility of that harm signal with these devices.
Two things resulted from these publications. First, this was an early example of long-term safety of these devices and demonstrated to the FDA how real-world evidence can be used adjunctively to help understand the safety concern that was seen in the Katsanos meta-analysis. Second, it gave me an opportunity to become more involved in evaluating the safety of these devices, including participation at the June 2019 FDA advisory meeting and developing the real-world evidence pathway to allow these devices to remain available in the US.
After the FDA panel, my partner Dr. Robert Yeh and I were asked to design and conduct a longitudinal surveillance mechanism using real-world evidence, which became the SAFE-PAD study. This was designed in collaboration with the FDA and provides biannual updates on the safety of these devices.
Why were you so active in working alongside the FDA?
As a practicing vascular interventionalist, I only knew how to practice in a world where I had drug-eluting stents and drug-coated balloons. My procedures felt incomplete not having these devices available for my patients. So, I wanted to get involved and understand the signal better because it was impacting my patients and my clinical practice. No one knew exactly who I was when I started publishing these papers. When I got the FDA’s attention, they started paying attention to some of the work that I was doing. One thing led to another. But, in the end, I’m a practicing clinician who cares about my patients, and I thought this was a critically important topic for something that deeply affects each of them.
What role did you see industry play in the paclitaxel update?
I think that industry partners—and Cook being at the front of them—really had a dramatic impact on how the paclitaxel pathway moved forward after the Katsanos analysis. This was a wonderful demonstration of how industry can work together to advocate for patient care. And that is exactly what the FDA wanted from industry. It was wonderful to see all of our industry partners we work with every day come together and put their competitiveness aside so that the patient remained at the center of the conversation. This was definitely one of the biggest positives to come out of the paclitaxel situation.
How did it help you as a physician when Cook analyzed our data and provided entire datasets?
I remember being with Cook at Charing Cross when the paclitaxel decision tool went live. I was immediately impressed with how Cook was willing to be so public and transparent with their data. Especially because we knew that the Zilver® PTX® trial was so complicated because of the randomization scheme.
In the 2019 letter, the FDA determined that only high-risk patients should be treated with paclitaxel-coated devices. Clinicians didn’t have a tool to identify those patients, so we were kind of lost. Cook stepped in with the novel prediction model that allowed us to discern who’s a good candidate for a paclitaxel device. I believe it helped physicians and Cook, who showed it’s a company that’s data-driven and doing things in the patient’s best interest.
Now that we can use paclitaxel more liberally again, the prediction model reminds me who’s at higher risk for a repeat event, enabling me to optimize my therapy and think about post-procedure care and monitoring. It also helps patients to be more aware of their own risks and benefits with the therapy. The prediction model gives real-world numbers based on the complexity of their disease and comorbidities.
For physicians who are still hesitant about paclitaxel, what level of data do you think they need to make them more comfortable?
Physicians who previously used paclitaxel-coated devices but stopped using them after the FDA letter should be comforted by the resounding amount of safety data that is now available. The data supports the safety of these devices exceeds five years, which is the farthest point that the Katsanos meta-analysis covered. For instance, we now have data out to eight years in SAFE-PAD. And we have additional demonstrations—both in randomized trials and observational studies—that show these devices are safe. The stamp of approval from the FDA further cements that this was a blip in the data and not a true causal relationship between paclitaxel and survival.
The other physician group that exists is the never-believers. They didn’t use drug therapy before Katsanos, and that gave them further reason not to use it. But I would remind them that there really are no other peripheral vascular devices that have as much randomized trial data as paclitaxel-coated devices. We use so many devices in our daily practice that are approved on single-arm trials or less, and we make many decisions based on anecdotal data. So, if you look at the totality of data for drug-coated devices, there is a continuous demonstration of patient benefit, not just binary stenosis on ultrasound, but reduction in clinical events that favors a patient outcome that should really resonate with anyone who’s truly thinking about their patients.
Where do you see the future of a drug coating other than paclitaxel?
We have not seen paclitaxel succeed below the knee yet. Maybe it’s the therapeutic agent and not how the agent’s being delivered. Or it could be the disease state that’s confounding this issue. Balloons with limus preparations below the knee have shown promise. Hopefully they will provide a pathway forward where paclitaxel has not been successful yet, at least not on a large scale.
- Secemsky EA, Shen C, Schermerhorn M, et al. Longitudinal assessment of safety of femoropopliteal endovascular treatment with paclitaxel-coated devices among Medicare beneficiaries: the SAFE-PAD study. JAMA Intern Med. 2021 Aug 1;181(8):1071-1080. doi: 10.1001/jamainternmed.2021.2738.
- Secemsky EA, Schermerhorn M, Carroll BJ, et al. Readmissions after revascularization procedures for peripheral arterial disease: a nationwide cohort study. Ann Intern Med. 2018 Jan 16;168(2):93-99. doi: 10.7326/M17-1058.






