Cook Medical is committed to helping treat patients who suffer from PAD, allowing patients to get back to living.
What is PAD?
What are the symptoms of PAD?
Most people with PAD don’t show any warning signs. Only one quarter to one third of people who are diagnosed with PAD have any symptoms at all.2 People who do have symptoms often mistake them for signs of aging.
Symptoms include:
- Leg pain when walking
- Numbness or weakness in the legs
- Aching pain in the feet or toes while at rest
- Ulcers or sores in the leg or foot that don’t heal
- Cold legs or feet
- Skin-color changes in the legs or feet

Who is at risk?
PAD can strike anyone, but it is most common in older people. PAD affects up to 20% of people over the age of 70.2 Smoking increases the risk of PAD. Heavy smokers are four times more likely than nonsmokers to develop PAD.2 On average, smokers are diagnosed with PAD 10 years earlier than nonsmokers.2
Risk factors include:
- Diabetes
- Obesity
- High blood pressure
- Lack of exercise
- Family history of hardening of the arteries (atherosclerosis)
- High cholesterol
Treatment of PAD
If you have PAD, your doctor will ask you to exercise more. If you are a smoker, your doctor will ask you to stop smoking. Your doctor may also prescribe drugs to thin your blood and to lower your cholesterol and blood pressure. All of these actions can help to slow the progression of PAD. They can also decrease your chances of having a heart attack or stroke. However, drugs and lifestyle changes alone don’t resolve all cases of PAD. For these patients, angioplasty, stenting, or bypass surgery may be necessary.

Angioplasty
A doctor can use a very small balloon to open up fatty deposits. The doctor uses a needle through the skin to access the artery and then inserts long, thin tools to reach fatty deposits. The doctor threads the deflated balloon through the fatty deposit and then inflates the balloon. After the fatty deposit is opened the doctor deflates and withdraws the balloon.
Stenting
If an artery is still too narrow after angioplasty, or your doctor thinks that the balloon treatment alone won’t help your artery stay open, he may use another tool called a stent. A stent is a small, metal tube that lines the inside of a fatty deposit and keeps the artery propped open. Like balloons, stents are delivered through blood vessels. Once a stent is placed it remains there forever. If an artery closes again, then another procedure may be necessary.
Bypass Surgery
Angioplasty and stenting doesn’t work for all patients. In these patients, doctors may do bypass surgery. Bypass surgery involves cutting open the leg and sewing a new vessel above and below the fatty deposit. The new vessel can be artificial or a vein that is transplanted from another part of the body.
What is Zilver PTX?
Zilver PTX is a drug-coated (drug-eluting) stent. The stent is used to treat the fatty deposits that can restrict blood flow in the largest artery of the thigh. This vessel is called the superficial femoral artery (SFA). The Zilver PTX stent is made of nitinol. Things that are made of nitinol return to their original shape after they are squeezed or bent. Nitinol makes the Zilver PTX stent open up by itself when your doctor puts it in your artery. Stents like Zilver PTX that open up by themselves are called self-expanding stents. Zilver PTX is the first stent with a drug coating that the FDA approved for use outside of the heart.
Benefits of Zilver PTX
Zilver PTX holds open the SFA and delivers the paclitaxel. The drug helps stop tissue growth that could close an artery. If an artery closes again, then another procedure may be necessary. This treatment is highly valued by patients because it improves their quality of life and decreases their need for repeat procedures.
Who should not get a Zilver PTX stent?
The following people should not get Zilver PTX:
- Women who are pregnant, are breast-feeding, or plan to become pregnant in the next five years.
- Patients who cannot take blood thinners (antiplatelet drugs).
- Patients in whom stents cannot be properly placed.
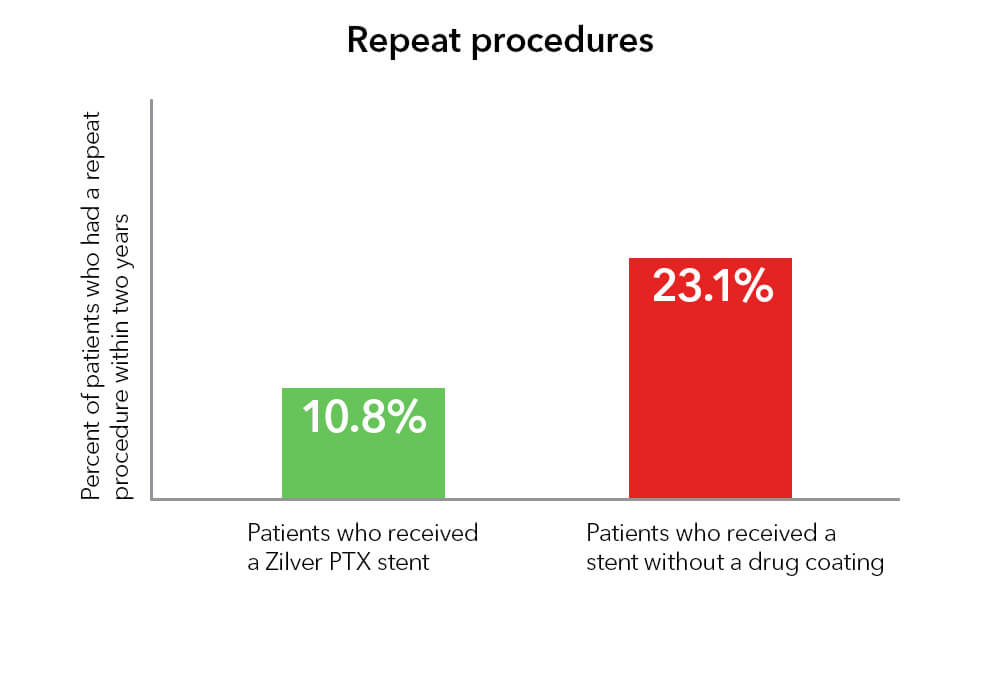
Repeat procedures
Compared with stents that are not coated with a drug, Zilver PTX reduced the number of patients who needed to have a repeat procedure by 53% at two years.3
What is paclitaxel?
Paclitaxel was discovered in 1967 in the bark of the Pacific yew tree. This drug blocks a cell’s ability to divide and is often used to treat cancer. For cancer patients, the paclitaxel dose is large and goes throughout the body. The Zilver PTX stent carries a much smaller paclitaxel dose that is applied directly to the artery wall. Your blood may wash away a small amount of the drug.
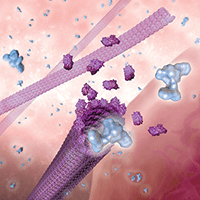
How does paclitaxel work?
The paclitaxel on Zilver PTX starts to go into your artery wall as soon as your doctor places the stent. Once inside a cell, paclitaxel blocks parts of cells that are involved in cell division. When the cells can’t divide, the excess growth that can close your artery again is prevented. Over time, a kind of cell growth that is good takes place. The inner lining of the artery grows over the stent and reduces the risk of blood-clot formation.
Risks of paclitaxel
The following could happen to you when you get a drug-coated stent:
- You could have an allergic reaction to paclitaxel.
- You could experience unwanted hair loss.

- Your number of red blood cells could decrease.
- Your bone marrow could produce fewer blood cells.
- You could need a blood transfusion.
- You could experience symptoms in your stomach and intestines.
- Your number of white blood cells or platelets could decrease.
- Your liver function could change.
- The wall of your artery could die or become damaged or inflamed.
- You could experience joint pain or muscle pain.
- Your nerves could become damaged.
After researching paclitaxel for more than 25 years, we have been able to conclude that paclitaxel from the Zilver PTX stent is no longer present in the body after approximately two months.5 Tens of thousands of patients have been treated with the Zilver PTX stent in the SFA for more than 10 years. We have commissioned numerous studies, including one of the world’s largest (479 patients) and longest (5-year follow-up) randomized controlled trials on a device to treat SFA disease.6 We believe such long-term results are critical to understanding how drug-eluting devices treat vascular disease.
- National Institutes of Health. Facts about peripheral arterial disease (P.A.D.). National Heart, Lung and Blood Institute Web site. https://www.nhlbi.nih.gov/health/educational/pad/docs/pad_extfctsht_general_508.pdf. Published August 2006. Accessed April 10, 2019.
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(suppl 1):S1-S75.
- Ansel G. Zilver PTX randomized trial of paclitaxel-eluting stents for femoropopliteal dis¬ease: 24-month update. Presented at: the Society for Cardiovascular Angiography and Interventions (SCAI) 2011; May 4-7, 2011; Baltimore, Maryland.
- Ask your doctor about the Zilver PTX Instructions for Use (IFU). This document contains full prescribing information, including indications, contraindications, warnings, precautions, and clinical data.
- Dake MD, Van Alstine WG, Zhou Q, et al. Polymer-free paclitaxel-coated Zilver PTX stents – evaluation of pharmacokinetics and comparative safety in porcine arteries. J Vasc Interv Radiol. 2011;22(5):603–610.
- Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation. 2016;133(15):1472-1483.