Effective 01 July 2024, C2Dx will take over sales, ordering, and distribution of these products in the US and Canada. At which point, please contact their Customer Service team via email at sales@C2Dx.com or call at 888-902-2239. Purchase orders can also be submitted via email to sales@C2Dx.com. All part numbers will remain the same.
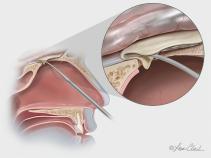
The Biodesign Duraplasty Graft is indicated for use as a dura substitute for the repair of dura mater. This graft is supplied sterile in peel-open packages and is intended for one-time use.
The products on this website are available for sale in the United States. Reference Part Numbers are not the same in all countries/regions. For products available in other countries, please choose one of our other websites from the region selector at the top of the site or contact your local Cook Medical representative.