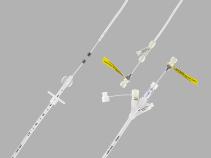
We regret to inform you that the Flourish® Pediatric Esophageal Atresia device is no longer commercially available. It’s important to note that the withdrawal of the Flourish device was not due to any safety or quality concerns. If you have questions, please contact a Flourish representative at FlourishHDE@CookMedical.com
Thank you.
The Flourish Pediatric Esophageal Atresia Device is indicated for use in lengthening atretic esophageal ends and creating an anastomosis with a nonsurgical procedure in pediatric patients, up to one year of age with esophageal atresia without a tracheoesophageal fistula (TEF), or in pediatric patients up to one year of age for whom a concurrent TEF has been closed as a result of a prior procedure. This device is indicated for atretic segments < 4 cm apart.
HUMANITARIAN DEVICE
Authorized by federal law for use in the treatment of lengthening atretic esophageal ends and creating an anastomosis with a nonsurgical procedure in pediatric patients, up to one year of age with esophageal atresia without a tracheoesophageal fistula (TEF), or in pediatric patients up to one year of age for whom a concurrent TEF has been closed as a result of a prior procedure. The effectiveness of this use has not been demonstrated.
This device requires IRB approval: H150003
The products on this website are available for sale in the United States. Reference Part Numbers are not the same in all countries/regions. For products available in other countries, please choose one of our other websites from the region selector at the top of the site or contact your local Cook Medical representative.
Specifications
| Order Number | Reference Part Number |
Instructions for Use (IFU) |
MR Status |
Oral Catheter Fr |
Oral Catheter Length cm |
Gastric Catheter Fr |
More Info |
|---|---|---|---|---|---|---|---|
| G47283 | FLRSH-PEA | — | N/A | 10 | 58 | 18 | Expand » |
|
Additional Specs
Description
-
Gastric Catheter Length cm
58
Magnet Diameter inch
0.25
|
|||||||
Wire Guide Diameter inch 0.035