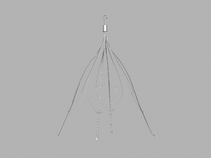
Used for the prevention of recurrent pulmonary embolism via placement in the vena cava in the following situations:
• Pulmonary thromboembolism when anticoagulant therapy is contraindicated;
• Failure of anticoagulant therapy in thromboembolic diseases;
• Emergency treatment following massive pulmonary embolism where anticipated benefits of conventional therapy are reduced; and
• Chronic, recurrent pulmonary embolism where anticoagulant therapy has failed or is contraindicated.
The products on this website are available for sale in the United States. Reference Part Numbers are not the same in all countries/regions. For products available in other countries, please choose one of our other websites from the region selector at the top of the site or contact your local Cook Medical representative.
Specifications
| Order Number | Reference Part Number |
Instructions for Use (IFU) |
MR Status |
Filter Max. Diameter mm | Filter Length mm | Introducer Sheath Fr | More Info |
|---|---|---|---|---|---|---|---|
| Femoral with NavAlign™ Delivery | |||||||
| G52917 | IGTCFS-65-1-FEM-TULIP |
|
|
30 | 50 | 7.0 | Expand » |
|
Additional Specs
Description
-
Introducer
Sheath Length cm
65
|
|||||||
| Jugular with NavAlign Delivery | |||||||
| G52916 | IGTCFS-65-1-JUG-TULIP |
|
|
30 | 50 | 7.0 | Expand » |
|
Additional Specs
Description
-
Introducer
Sheath Length cm
65
|
|||||||
| UniSet with NavAlign Delivery | |||||||
| G52918 | IGTCFS-65-1-UNI-TULIP |
|
|
30 | 50 | 7.0 | Expand » |
|
Additional Specs
Description
-
Introducer
Sheath Length cm
65
|
|||||||