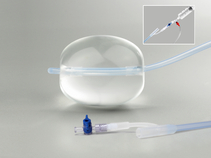
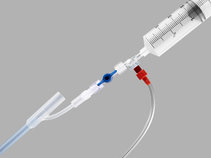
Used to provide temporary control or reduction of postpartum uterine bleeding when conservative management is warranted.
- Can be used following vaginal or cesarean delivery.
- Includes rapid instillation components to facilitate inflation of the balloon.
- May be used with B-lynch compression sutures if clinically warranted.
- Is constructed of latex-free silicone.
- Includes a syringe, a dual check valve, and 180 cm of tubing with attached bag spike.
The products on this website are available for sale in the United States. Reference Part Numbers are not the same in all countries/regions. For products available in other countries, please choose one of our other websites from the region selector at the top of the site or contact your local Cook Medical representative.