An overview of advanced imaging methods for the diagnosis and treatment of type B aortic dissection.
BY DARREN KLASS, MBChB, MD, MRCS, FCRC, FRCPC
Imaging of aortic dissection can be performed both periprocedurally with computed tomography (CT) and intraprocedurally with transesophageal echocardiography (TEE) and subtraction angiography. Although there are limitations to each modality, overall, these methods are capable of demonstrating the pathology, allowing for a diagnosis to be made, a procedure to be performed, and follow-up to be completed with relative ease.
Aortic dissection is an extremely complex disease, often with multiple fenestrations along the length of the intimomedial flap; many of these are large and therefore pose potential pitfalls regarding passage of wires and stent grafts inadvertently into the false lumen, with potentially catastrophic consequences. In addition to the complexities encountered intraprocedurally, assessment of the morphology of the dissection and aorta at the time of diagnosis is essential to ensure the correct management strategy is undertaken. If a decision is made to proceed with endovascular repair of the dissection with stent grafts, accurate measurement of the landing zone is required to confirm appropriate stent graft sizing to minimize the risk of a retrograde dissection due to device oversizing. This seemingly simple task can be relatively inaccurate if the appropriate imaging protocols are not followed and setup of the CT scanner is not optimized.
The aim of this article is to highlight the advanced imaging techniques available that aid in diagnosis, treatment planning, procedural execution, problem solving, and follow-up.
COMPUTED TOMOGRAPHY
CT angiography is a fast, reliable, and reproducible method of assessment of acute aortic syndromes. The technology has advanced rapidly, and the speed at which images are acquired has significantly improved. Many CT scanners can acquire images using two separate imaging sources (dual energy), which in turn allow a patient to move much faster through the scanner, obtaining whole body scans with submillimeter resolution in a matter of milliseconds.1,2
The combination of rapid patient movement through the CT scanner and the dual energy acquisition have allowed imaging of the aorta to be acquired between two heart beats, which then images the ascending aorta with little or no motion. The coronary cusps, ascending aorta, and transverse arch are free from artifact, so diagnosing a subtle type A dissection becomes much easier for the clinician and can be made with confidence (Figure 1A). If this imaging protocol is utilized, it negates the need for a confirmatory TEE in many cases. The lack of motion of the aorta during image acquisition improves the resolution and allows for accurate measurement of the aorta, as the wall is easy to identify (Figure 1B). In cases of acute and subacute dissection, where oversizing can lead to a retrograde dissection with stent graft deployment, utilizing this technique allows measuring and planning with much more confidence and accuracy than standard image acquisition.
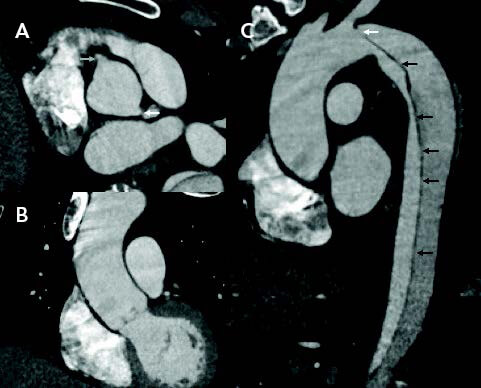
Figure 1. CT images using an ultrahigh-pitch protocol demonstrating well-visualized coronary origins (arrows) with no aortic motion (A); motionless ascending aorta, aiding in excluding a dissection and improving measurement accuracy (B); and the entry tear (white arrow) and multiple tiny fenestrations along the entire length of the intimomedial flap (black arrows) (C).
This technique is termed ultrahigh-pitch CT, where the patient is moved through the scanner much faster than a conventional scan. Ultrahigh-pitch CT scanning is possible on modern scanners due to the speed at which images are acquired, the computational power of the processors, as well as the ability to utilize electrocardiography (ECG) gating.
ECG gating allows the scanner to initiate image acquisition at particular stages of the cardiac cycle. The patient is monitored via ECG leads and the software identifies the QRS complex and triggers between the complexes to decrease motion as much as possible. This method of image acquisition has a temporal resolution of approximately 75 ms, which allows for a full high-resolution cardiac CT scan in 250 ms.
This not only allows for motion reduction in the ascending aorta but also a much more sensitive and specific image where submillimeter fenestrations can be identified (Figure 1C), which may influence decisions regarding stent graft length.
In addition, modern CT scanners allow for time-resolved imaging, where a small volume of tissue can be interrogated in real time, with the scanner moving the patient backward and forward over a short distance (15– 25 cm) to assess for dynamic changes in contrast flow. Contrast can be imaged throughout the cardiac cycle in the arterial and delayed phase, allowing an angiographic rendering with significantly better soft tissue assessment and longer scan times, as well as relatively low doses of contrast (typically 50 mL) and radiation.
MAGNETIC RESONANCE IMAGING
Historically, magnetic resonance imaging (MRI) has not been widely utilized in aortic imaging due to the lack of robust protocols and adequate expertise for interpretation. MR angiography has evolved with the design of parallel imaging protocols, which allow simultaneous imaging of multiple slices of tissue, decreasing scan times by up to eight orders of magnitude.3 A main benefit of MRI of the aorta is that it carries no dose penalty and therefore is the ideal screening and follow-up tool for patients requiring follow-up of aortic pathology for life (eg, those with connective tissue disorders) or for screening patients with risk factors for aortic pathology. Many of the examinations for screening and follow-up can be performed via MRI without the use of gadolinium contrast agents, as evaluation of only interval change in the size of the aorta is often required, and the soft tissue resolution and signal-to-noise ratio are more than adequate for answering these simple questions and are superior to noncontrast CT for basic follow-up. If there is any progression in the aortic disease, including an increase in size, the patient can then be imaged with contrast-enhanced CT for procedure planning.
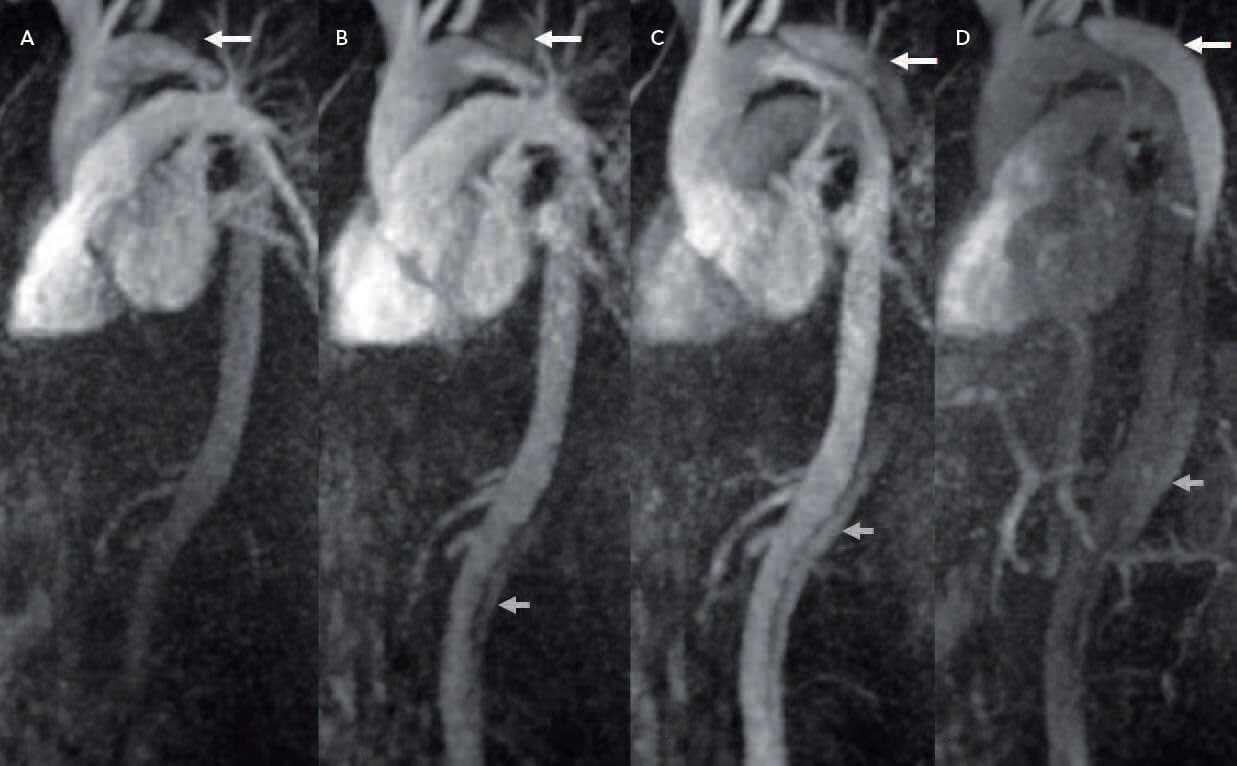
Figure 2. Time-resolved MR angiography demonstrating very early antegrade filling of the false lumen (arrow) (A), with further antegrade filling (B), and both antegrade and retrograde filling with altered signal in each due to the flow differential (C, D).
Although MRI plays little or no role in the diagnosis of acute aortic syndromes because it is time consuming and requires specialist radiology training to develop protocols and interpret images, it does play an important role in problem solving for complex patients. The use of time-resolved MRI allows for the dynamic assessment of blood flow after the administration of gadolinium. This assessment often can be performed with half the dose of gadolinium required for conventional imaging and provides detailed information to the clinician on flow dynamics in the aorta.4 As opposed to four-dimensional MR flow assessment, time-resolved MRI cannot quantify flow but does provide information on the direction of flow and enhancement. The temporal resolution of the imaging protocol can be adjusted where the scan volume is sampled more or less frequently depending on the question asked. This can be particularly helpful after stenting for dissection, where the direction and speed of flow into the false lumen can be of particular importance for patients in whom there is the question of antegrade flow in a false lumen after stenting (Figure 2) and earlier intervention would be preferred.
A second potential use of time-resolved MRI is for perfusion assessment of end organs, such as the kidneys in patients with resistant hypertension after an aortic dissection. The study can be performed and the kidneys imaged throughout the arterial, corticomedullary, and excretory phases to assess for comparative enhancement of the kidneys and the presence of a delayed nephrogram, which would suggest altered perfusion to that kidney.
Delayed imaging of the aorta at 2 to 5 minutes following contrast administration allows for high-resolution imaging with a spatial resolution of 1 mm. Any slow, delayed filling of the false lumen, which may be missed on CT imaging traditionally at 90 seconds, can be evaluated using a fast breath-hold sequence or a high-resolution sequence. Delayed vascular imaging is referred to as steady-state imaging as the contrast reaches equilibrium in the arterial and venous systems.
INTRAVASCULAR ULTRASOUND
Intravascular ultrasound (IVUS) provides high-resolution, real-time imaging of the vascular system and has become an increasingly recommended standard of care when treating aortic dissection. A number of vendors produce IVUS transducers with either radial 360° array transducers (Visions PV .035, Philips) or side facing transducers.
Each configuration has its unique aspects. The IVUS catheter, for instance, is very suitable for image-guided intervention and can be oriented and directed with the side-facing transducer, as the transducer provides a limited visual field in the direction of the transducer only. The operator can therefore turn the IVUS catheter in the direction of the intended intervention. If a 360° view is required, the operator is able to visualize the entire aorta on a single image; however, it is more difficult to direct the catheter.
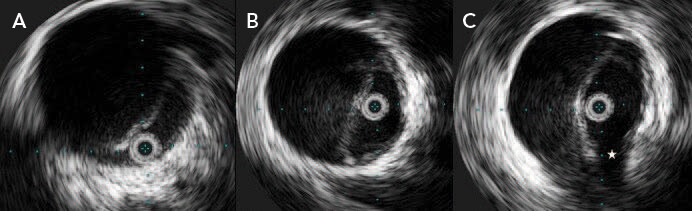
Figure 3. IVUS demonstrating the mobility of the intimomedial flap in systole with compression of the true lumen (A), diastole with expansion of the true lumen (B), and near-complete delamination of the intimomedial flap and extension into the left renal artery ostium (star) with a dynamic obstruction (C).
The radial 360° array transducer allows for a circumferential view of the aorta (Figure 3). When the exact orientation of vessels is known, such as the visceral and renal vessels, the catheter can be appropriately oriented. The 360° array transducer is well suited to aortic imaging, as the entire aorta and dissection can be evaluated in each image in real time. The movement of the intimomedial flap can be evaluated (Figure 3A and 3B), particularly in dynamic obstruction of branch vessels (Figure 3C). The morphology of the flap can be assessed, and most importantly, the path of the wire can and should be assessed throughout the path of the wire to ensure that when stenting is performed, the stent is placed in the true lumen. Without IVUS guidance, it is possible to place the wire in the true lumen distally and for the wire to traverse multiple fenestrations into the false lumen and back into the true lumen without the knowledge of the operator, with potentially catastrophic consequences. The use of IVUS allows accurate navigation of the wire through the true lumen of the access vessel to the ascending aorta for stenting.
A further benefit of IVUS is the ability to measure the diameter of the aorta and lumina prior to stenting. This is of particular importance if the initial diagnostic images were not obtained using ultrahigh-pitch CT and motion may have precluded accurate measurement of the landing zone. The transducer used for aortic imaging is placed over a 0.035-inch wire and has a field of view of 5 cm in diameter. This catheter (Visions PV .035) requires a 9-F sheath for access and is a single-use item.
TRANSESOPHAGEAL ECHOCARDIOGRAPHY
TEE has been used extensively in diagnosing acute aortic pathology and can be used intraoperatively to aid in guidance of the wire in the true lumen. However, this is limited to the thoracic aorta and therefore the abdominal path of the wire cannot be assessed. This modality, with the increased use of IVUS, has become an adjunct imaging tool for the endovascular operator and also can be used to assess for flow in the false and true lumina following stenting, as some IVUS systems do not have Doppler capability. TEE also has a role in the workup of acute aortic syndromes when the CT is equivocal, particularly in the diagnosis of type A dissection or when a type A dissection is suspected in patients with renal failure and iodinated contrast agents should be avoided.
CONCLUSION
Imaging has rapidly advanced and the ability to obtain high-resolution, accurate imaging of the entire aorta with little motion is not only possible but can be done without the need for ß blockade. It is necessary for any physician treating aortic dissection to become familiar with advanced imaging options and incorporate them into the workup, treatment, and follow-up protocols for patients with aortic dissection.
Darren Klass, MBChB, MD, MRCS, FCRC, FRCPC
Department of Radiology
Division of Interventional Radiology
University of British Columbia
Vancouver, British Columbia, Canada
darren.klass@vch.ca
Disclosures: Proctor, speaker, and consultant for Cook Medical.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266-e369.
- Ueda T, Chin A, Petrovitch I, Fleischmann D. A pictorial review of acute aortic syndrome: discriminating and overlapping features as revealed by ECG-gated multidetector-row CT angiography. Insights Imaging. 2012;3:561-571.
- Vessie EL, Liu DM, Forster B, et al. A practical guide to magnetic resonance vascular imaging: techniques and applications. Ann Vasc Surg. 2014;28:1052-1061.
- Thakor AS, Chung J, Patel P, et al. Use of blood pool agents with steady-state MRI to assess the vascular system. J Magn Reson Imaging. 2017;45:1559-1572.
