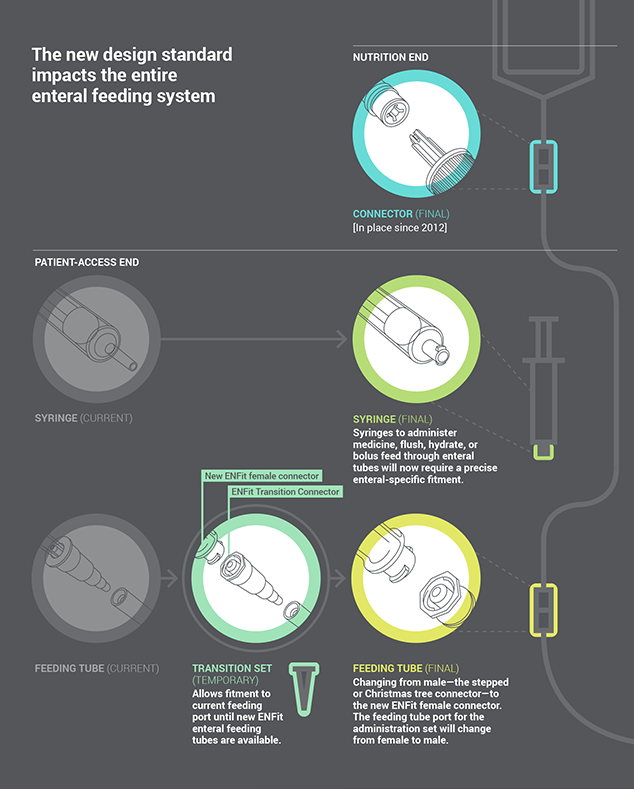
Tom Hancock, a founder and executive director of the Global Enteral Device Supplier Association (GEDSA), further discusses the new design standard and its impact on the entire enteral feeding system.
Visit gedsa.org for more information on GEDSA
The Channel: What can you share with the readership regarding the deadlines
and objectives related to the transition?
There is one hard and fast deadline: July 2016. This deadline has been set
by California legislation (Senate Bill 158; Assembly Bill 1867) that prohibits the use of intravenous, epidural or enteral feeding connections that would fit into a connection port other than the type for which it was intended.
July 2016 is a hard deadline that our industry interprets as the market should be fully transitioned by then. That means we have to be way ahead of it in order to carefully transition the market. Technically, that’s the only hard, fast deadline. The International Organization for Standardization (ISO) will set a loose deadline of three years after the official publication of the standard.
We, as an industry, because of the California legislation, are trying to get ahead of the published standard and so the California deadline supersedes the ISO standard and the publication. The other thing is that manufacturers view the conversion of California and the United States as the same, so the industry will not convert California as separate, different than other markets. Logistically, it would be incredibly challenging to manage the supply chain with two sets of products.
The Channel: Going forward, how does all the collaborative effort in this transition address patient and healthcare provider safety concerns?
I think that is really important. These enteral devices have to be able to connect to each other in order to have a safe environment for patients. Most importantly, they have to not connect with things they are not supposed to. The ISO design addresses that issue; however, the piece that is not discussed within the ISO process is the implementation piece. How are you supposed to do this and with unintended consequences?
Therefore, every single member and manufacturer and just about every clinician you speak to will say, “This is awesome. This is great that we are finally addressing this issue.” But it doesn’t come without a significant risk since, by design, the old connectors are not supposed to connect with the new connectors. There’s a transition plan that we have temporarily put into place to make sure there is backwards compatibility.
If manufacturers didn’t work together to: a) come up with a transition plan, b) decide to introduce the new connectors in the same time period and c) to make sure we are all saying the same thing, we would confuse our customers. If there weren’t that type of collaboration, it would be mass confusion and worse. It would lead to disruption of therapy. The biggest single driver is patient safety, ensuring that we carefully transition the market without disruption
of therapy.
So collaboration between the manufacturers is really important, as is collaboration with the various associations. We have been able to make a great connection with the folks at A.S.P.E.N., the American Society for Parenteral and Enteral Nutrition. That organization and in particular, Dr. Peggi Guenter has been a huge proponent of our efforts. They have helped lead the development of our FAQs and other training tools as well as lead the charge presenting these changes at various conferences and webinars.
The Joint Commission has been working with us and they recently released Sentinel Event Alert #53 to demonstrate the importance if these changes. The FDA is also available to provide guidance and answer questions for us. The Institute for Safe Medication Practices has also been strong contributor as has the Association for Advancement of Medical Instrumentation.
The Oley Foundation is another great collaborator. They are a patient care advocacy group and they are a very strong voice within that community. We are working with them to make sure that we have the right transition plan in place and we are addressing their issues and doing studies to make sure that when we make the transition it is suitable. They, too, are helping get the word out.
For medication administration concerns, we are working with the American Society of Health-System Pharmacists (ASHP). We have participated in their Medication Safety Collaborative, where we did a talk and a breakout session for hands-on training. So we are going to continue to foster that relationship to get the word out to pharmacists in the hospital setting. We are also working with the Walgreens of the world that deliver home infusion. They will also communicate the message through to their pharmacies at the community level.
So the collaboration just continues to build and we are continuing to create relationships with various associations that represent the various personnel and functions that we need to try to reach through hospitals, long-term care and home care settings.
Click here to view Part One of this series ,”Get to know GEDSA”