Acute gastrointestinal bleeding can be a difficult and challenging condition. Cook is constantly testing and evaluating the efficacy and safety of our products, like the Hemospray® Endoscopic Hemostat, which creates a mechanical barrier over bleeding sites. As you will see below, the recent publication of the largest multicenter international registry of Hemospray patients shows high hemostasis rates and rebleed rates that are in keeping with or lower than predicted rates. The registry also investigates the efficacy of Hemospray as monotherapy, as combination therapy and as rescue therapy.
The full registry has been e-published and is available online at: https://onlinelibrary.wiley.com/doi/abs/10.1111/den.13502
Outcomes from an international multicenter registry of patients with acute gastrointestinal bleeding undergoing endoscopic treatment with Hemospray*†
Prospective data from 314 patients across 12 centers. “These data show high rates of immediate hemostasis overall and in all subgroups. Hemospray was effective in achieving primary hemostasis with an overall hemostasis rate of 89.5%”
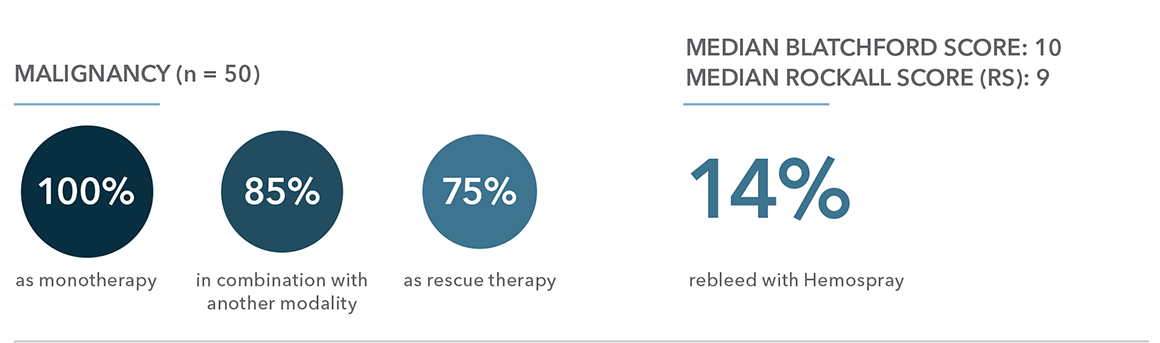

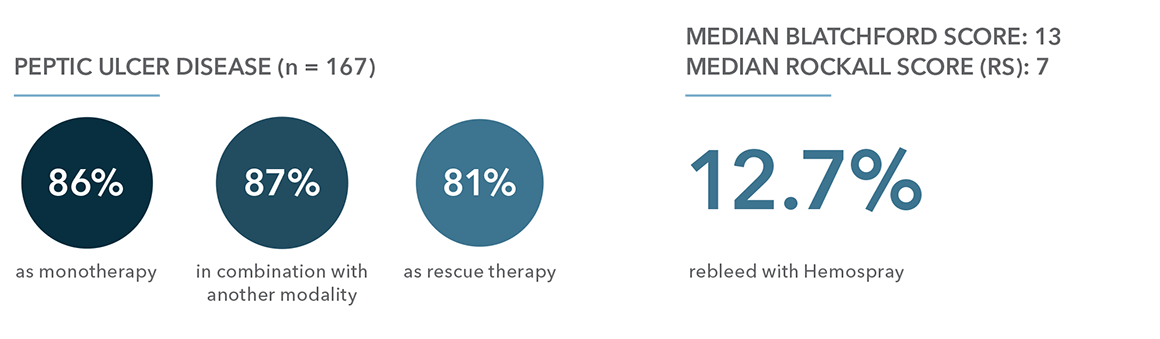
Monotherapy: use of Hemospray on its own.
Combination: use of Hemospray with conventional modalities (adrenaline injection, thermocoagulation and mechanical clips) as an adjunct therapy to a single modality to help achieve hemostasis or as an adjunct with two other modalities after successful hemostasis.
Rescue therapy: use of Hemospray when all other conventional modalities failed to achieve hemostasis in the same endoscopic session.
“Our study was able to show that Hemospray in combination with conventional modalities had a lower rebleeding rate and all-cause mortality rate than monotherapy and rescue therapy”
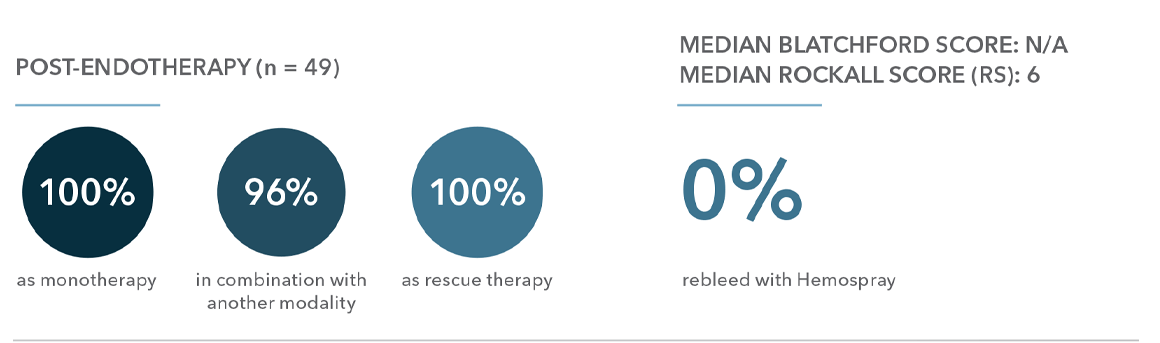
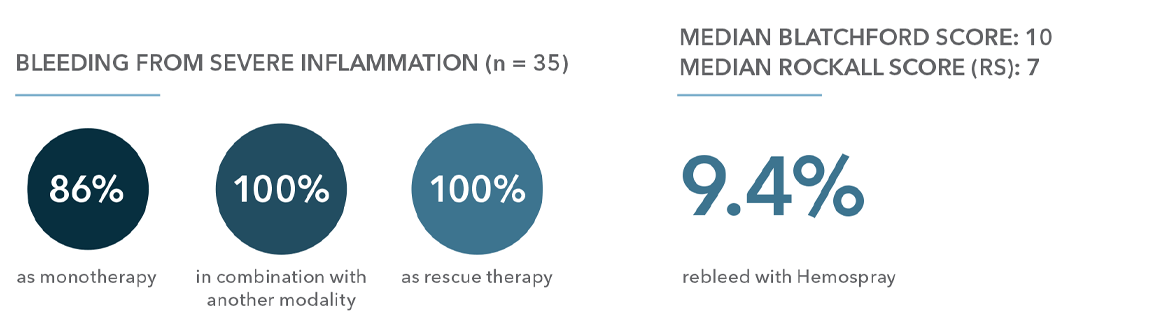
“Conclusion: These data show high level of immediate hemostasis overall and in all subgroups. Rebleeding and mortality rates were in keeping / lower than predicted rates.”
