Bloomington, Ind. — Just weeks after the Food and Drug Administration (FDA) approved Cook Medical’s Zilver© PTX© Drug-Eluting Peripheral Stent, Riverside Methodist Hospital in Columbus, Ohio, has treated the first patient with the device as part of Cook’s U.S. commercial launch.
“It was a great honor for my institution to be first to implant Cook Medical’s Zilver PTX as part of the stent’s commercial roll-out,” said Gary Ansel, M.D., director for the Center for Critical Limb Care at Riverside Methodist Hospital. “This technology is so advanced and offers such prolonged patient benefit, I believe it will very quickly challenge older PAD treatments such as balloon angioplasty and bare metal stenting in the U.S. as the standard of PAD care.”
Zilver PTX, approved for use in the above-the-knee femoropopliteal artery, is the only drug-eluting stent approved for use in a peripheral artery in the U.S. The device has a proven drug effect 1 that reduces by more than 50 percent the need for followup procedures to reopen the artery. These followup procedures can be expensive, which places extra burdens on patients, physicians and facilities. 2
“The first commercial use of this stent represents what I think will be the start of a complete shift in the way physicians will treat PAD patients in this country,” said Rob Lyles, vice president and global leader of Cook Medical’s Peripheral Intervention division. “Zilver PTX, the only FDA-approved stent that brings the proven benefits of drug elution to the peripheral arteries, is an effective treatment option for patients who suffer with the painful and potentially debilitating consequences of PAD. We are extremely proud to bring this technology to American doctors and the patients they treat.”
“The first commercial use of this stent represents what I think will be the start of a complete shift in the way physicians will treat PAD patients in this country”
This unique combination therapy device combines the mechanical support of stenting with the drug paclitaxel, which limits cell growth that can reclog the artery. The combination has been shown to maintain arterial blood flow to the superficial femoral artery (SFA) in seven out of ten patients through 24 months after implantation. 3
The disease Zilver PTX targets, peripheral arterial disease (PAD), is one of the fastest-growing and most pervasive diseases of our time, affecting an estimated 8-12 million Americans each year. 4 PAD is a disease in which plaque builds up in the arteries, which carry blood to the head, organs and limbs. While PAD typically affects arteries in the legs, it can also affect blood flow to the head, arms, kidneys and stomach. 5 People aged 50 and over who have a history of diabetes, smoking and high blood pressure are at higher risk for PAD. 6 Other treatment modalities for PAD include medical management, lifestyle changes such as exercise, balloon angioplasty, bare metal stenting and surgical bypass of the blocked artery.
Zilver PTX is currently available in 54 markets, including the European Union, Brazil, Australia, Taiwan and Japan. Thousands of patients worldwide have been treated with Cook’s device. 7
Dr. Gary Ansel is a principal investigator in the global Zilver PTX clinical trial. He is a paid consultant to Cook Medical with respect to its medical devices.
About Cook Medical
A global pioneer in medical breakthroughs, Cook Medical is committed to creating effective solutions that benefit millions of patients worldwide. Today, we combine medical devices, drugs, biologic grafts and cell therapies across more than 16,000 products serving more than 40 medical specialties. Founded in 1963 by a visionary who put patient needs and ethical business practices first, Cook is a family-owned company that has created more than 10,000 jobs worldwide. For more information, visit www.cookmedical.com. Follow Cook Medical on Twitter and LinkedIn.
1 Dake MD, Van Alstine WG, Zhou Q, et al. Polymer-free paclitaxel-coated Zilver PTX stents—evaluation of pharmacokinetics and comparative safety in porcine arteries. J Vasc Interv Radiol. 2011;22(5):603-610.
2 Burket MW. The economic impact of restenosis and the economics of drug elution. Presented at: Vascular Interventional Advances (VIVA) 2011; October 18-21, 2011; Las Vegas, NV.
3 Ansel G. Zilver PTX randomized trial of paclitaxel-eluting stents for femoropopliteal disease: 24-month update. Presented at: the Society for Cardiovascular Angiography and Interventions (SCAI) 2011; May 4-7, 2011; Baltimore, MD.
4 National Institutes of Health Peripheral Arterial Disease Fact Sheet. NIH Publication No. 06-5837. 2006.
5 National Institute of Health, National Heart Lung and Blood Institute. What is peripheral arterial disease?http://www.nhlbi.nih.gov/health/health-topics/topics/pad/. Accessed August 10, 2012.
6 National Institute of Health, National Heart Lung and Blood Institute. Who is at risk for peripheral arterial disease?http://www.nhlbi.nih.gov/health/health-topics/topics/pad/atrisk.html. Accessed October 15, 2012.
7 Data on file.
Bloomington, Ind. — Reinforcing its commitment to advancing interventional techniques and technologies to treat disease, Cook Medical is pleased to announce a new contract with leading health care supply chain and contracting company Novation for Cook’s peripheral intervention and peripheral diagnostic devices.
Cook peripheral vascular products under this contract will be offered to the more than 65,000 members and affiliates of VHA Inc., UHC, Children’s Hospital Association and Provista, all served by Novation.
The contract, which goes into effect Jan. 1, 2013, will cover devices in Cook’s peripheral vascular intervention and diagnostics portfolio, which include access and biopsy needles, balloons, balloon stents, diagnostic wire guides and catheters, interventional wire guides, embolization coils, self-expanding stents and vena cava filters.
“We are pleased to continue our strong partnership with Novation,” said David Reed, vice president of HBS at Cook Medical. “As our portfolio of minimally invasive peripheral vascular products grows, comprehensive contracts like this one with Novation are critical to our ability to reach a diverse group of institutions and make our technology widely accessible, particularly to academic medical centers, which we think play a valuable and important role in health care.”
About Novation, Winner of the Ethics Inside® Certification
Founded in 1998, Novation is the leading health care supply chain expertise and contracting company for the more than 65,000 members of VHA Inc. and UHC, two national health care alliances, Children’s Hospital association, an alliance of the nation’s leading pediatric facilities, and Provista, LLC. Novation provides alliance members with sourcing services, as well as information and data services. Based in Irving, Texas, Novation develops and manages competitive contracts with more than 600 suppliers. VHA, UHC, and Provista members used Novation contracts to purchase more than $40 billion in 2011. Novation recently earned the coveted Ethics Inside Certification from Ethisphere Institute, a leading international think tank dedicated to the research and promotion of best practices in corporate ethics and compliance. Novation was also named on Ethisphere’s World’s Most Ethical Companies list, and is the only company in the health care industry to earn both distinctions. To learn more about Novation, please visit the newly designed novationco.com and follow @NovationNews.
About Cook Medical
A global pioneer in medical breakthroughs, Cook Medical is committed to creating effective solutions that benefit millions of patients worldwide. Today, we combine medical devices, drugs, biologic grafts and cell therapies across more than 16,000 products serving more than 40 medical specialties. Founded in 1963 by a visionary who put patient needs and ethical business practices first, Cook is a family-owned company. For more information, visit www.cookmedical.com. Follow Cook Medical on Twitter and LinkedIn.
Bloomington, Ind. — Cook Medical has received U.S. Food and Drug Administration (FDA) marketing approval for the first devices in its Zilver® PTX® Drug-Eluting Peripheral Stent portfolio, company officials reported today. It’s the first time the FDA has approved a drug-eluting stent to treat blockages in a peripheral artery.

“This approval marks the start of Cook’s program to bring the benefits of drug elution to U.S. physicians treating the peripheral arteries,” said Rob Lyles, vice president and global leader of Cook Medical’s Peripheral Intervention division.“No other company can match Cook’s commitment to this technology, and by the end of 2013, we expect to have a full suite of drug-eluting peripheral stents in the most commonly used lengths and diameters available to U.S. physicians.”
“This approval marks the start of Cook’s program to bring the benefits of drug elution to U.S. physicians treating the peripheral arteries”
The Zilver PTX Drug-Eluting Stent is intended to treat peripheral arterial disease (PAD) in the superficial femoral artery (SFA).
In order to supply as many physicians as possible with this new technology, Cook is making Zilver PTX available initially in 80 mm lengths in 6 mm and 7 mm diameters. The products indications for use also allow two Zilver PTX 80 mm stents to be overlapped to treat longer lesions up to 140 mm. The FDA approval also includes 40 mm and 60 mm lengths, which will be introduced to the U.S. early in 2013. Cook expects to receive regulatory approval for 120 mm length stents in both diameters next year.
Data from Cook’s pivotal clinical trial indicate:
Eight out of ten patients treated with Zilver PTX still had open arteries (primary patency) after one year1. That compares to only 3 out of 10 patients treated with angioplasty alone. Patients who received a bare metal stent required more than twice as many reintervention procedures to reopen the SFA as patients who received Zilver PTX.2 “After conducting the largest randomized controlled study of peripheral stenting ever undertaken, we now see remarkable results in patients treated with Zilver PTX,” said Michael Dake, M.D., a professor in the Department of Cardiothoracic Surgery at Stanford University School of Medicine and medical director of the Cath/Angio Laboratories at Stanford Medical Center, Palo Alto, Calif.
“With this approval, treating PAD in the U.S. will begin to undergo the same revolution that drug elution did for treating coronary artery disease,” added Gary Ansel, M.D., director for the Center for Critical Limb Care at Riverside Methodist Hospital in Columbus, Ohio, and an assistant clinical professor of medicine in the Department of Internal Medicine at the University of Toledo Medical Center in Toledo, Ohio. “Drug-eluting stents such as Zilver PTX will move quickly, in my opinion, to become the standard of care for PAD patients worldwide.”
Cook’s Zilver PTX stent is already approved for sale in more than 50 markets, including the European Union, Japan, Brazil and most of South America, Australia, New Zealand and Taiwan. The device is being introduced to the U.S. market in a five-step process designed to make this technology available to as many patients as possible initially.
(Drs. Dake and Ansel, who served as global principal investigators for the Zilver PTX clinical trial, are paid consultants to Cook Medical with respect to its medical devices.)
About Zilver PTX
How does Zilver PTX work?
A physician gains arterial access through the groin and guides a Zilver PTX stent to the narrowed artery with a catheter. The stent is deployed and expands like a scaffold to help keep the artery open after the catheter is withdrawn. The drug paclitaxel, which coats the stent, is taken up by the cells of the arterial wall to help prevent the renarrowing of the artery over time.
What are the main features of the device?
A combination therapy device, Zilver PTX both restores patency (blood flow) and provides targeted delivery of paclitaxel, a cell growth-limiting drug proven to reduce arterial restenosis (post-procedural blockages). This drug coats the stent without the use of a polymer, eliminating risks that may arise directly from a polymer. Zilver PTX is made of nitinol, a“shape memory” metal alloy, and is engineered to withstand the dynamic forces of the superficial femoral artery (SFA). Zilver PTX is the first peripheral vascular device that combines the mechanical support of stenting with the drug paclitaxel to reduce the risk of restenosis.
What data supports the efficacy of Zilver PTX?3
Two-year data from the Zilver PTX Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease indicate that the stent demonstrated 74.8 percent primary patency at 24 months in the PTX group, compared to just 57.8 percent for patients with optimal percutaneous transluminal angioplasty and bare metal stents in the 479 patient study.
What are other current means of treatment for PAD?4
Current treatment options for PAD include lifestyle changes, medication, exercise, angioplasty, bare metal stenting and bypass surgery.
What is Zilver PTX indicated for and what are the risks and contraindications for this device?
INDICATIONS: indicated for improving luminal diameter for the treatment of de novo or restenotic symptomatic lesions in native vascular disease of the above-the-knee femoropopliteal arteries having reference vessel diameter from 4mm to 7mm and total lesion lengths up to 140 mm per limb and 280 mm per patient. CONTRAINDICATIONS: Women who are pregnant, breastfeeding, or plan to become pregnant in the next 5 years should not receive a Zilver PTX Drug-Eluting Peripheral Stent. Patients who cannot receive recommended anti-platelet and/or anti-coagulant therapy. Patients judged to have a lesion that prevents proper placement of the stent or stent delivery system. WARNINGS: Persons with allergic reactions to nitinol may suffer an allergic reaction to this implant • Persons allergic to paclitaxel may suffer an allergic reaction to this implant • The safety and effectiveness of implanting more than four Zilver PTX Drug Eluting Peripheral Stents in a patient has not been clinically evaluated. PRECAUTIONS: To avoid involvement of the common femoral artery, the proximal end of the stent should be placed at least 1 cm below the origin of the superficial femoral artery. To avoid involvement of the below-the-knee popliteal artery, the distal end of the stent should be placed above the plane of the femoral epicondyles • This product is intended for use by physicians trained and experienced in diagnostic and interventional vascular techniques. Standard techniques for interventional vascular procedures should be employed • Manipulation of the Zilver PTX Drug-Eluting Peripheral Stent requires fluoroscopic control • Do not try to remove the stent from the introducer system before use • Ensure that the red safety lock is not inadvertently removed until final stent release • Deploy the stent over an extra stiff or ultra stiff wire guide • Do not push the hub toward the handle during deployment • Do not expose the delivery system to organic solvents (e.g., alcohol) • Do not use power injection systems with the delivery system • Do not rotate any part of the system during deployment • The device is intended for single use only. Do not resterilize and/or reuse this device • Repositioning of the device after deployment is not possible since the introducer catheter cannot be re-advanced over the stent once deployment begins. POTENTIAL ADVERSE EVENTS:Potential adverse events that may occur include, but are not limited to Allergic reaction to anticoagulant and/or antithrombotic therapy or contrast medium • Allergic reaction to nitinol • Arterial aneurysm • Arterial rupture • Arterial thrombosis • Arteriovenous fistula • Atheroembolization (Blue Toe Syndrome) • Death • Embolism • Hematoma/hemorrhage • Hypersensitivity reactions • Infection • Infection/abscess formation at access site • Ischemia requiring intervention (bypass or amputation of toe, foot or leg • Pseudoaneurysm formation • Renal failure • Restenosis of the stented artery • Stent embolization • Stent malapposition • Stent migration • Stent strut fracture • Vessel perforation or rupture • Worsened claudication/rest pain. Paclitaxel: Although systemic effects are not anticipated, refer to the Physicians’ Desk Reference for more information on the potential adverse events observed with paclitaxel. Potential adverse events, not described in the above source, may be unique to the paclitaxel drug coating, including • Allergic/immunologic reaction to the drug coating • Alopecia • Anemia • Blood product transfusion • Gastrointestinal symptoms • Hematologic dyscrasia (including leukopenia, neutropenia, thrombocytopenia) • Hepatic enzyme changes • Histologic changes in vessel wall, including inflammation, cellular damage, or necrosis • Myalgia/Arthralgia • Myelosuppression • Peripheral neuropathy
See package insert for full product information.
About Cook Medical
A global pioneer in medical breakthroughs, Cook Medical is committed to creating effective solutions that benefit millions of patients worldwide. Today, we combine medical devices, drugs, biologic grafts and cell therapies across more than 16,000 products serving more than 40 medical specialties. Founded in 1963 by a visionary who put patient needs and ethical business practices first, Cook is a family-owned company. For more information, visit www.cookmedical.com. Follow Cook Medical on Twitter and LinkedIn.
1,2Dake M., et. Al, Paclitaxel-Eluting Stents Show Superiority to Balloon Angioplasty and Bare Metal Stents in Femoropopliteal Disease: Twelve Month Zilver PTX Randomized Study Results. Circulation: Cardiovascular Interventions, August 5, 2011
2Dake M. Zilver PTX Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease: Two Year Results, Presented at International Symposium on Endovascular Therapies (ISET), January 17, 2011, Miami Beach, Florida
3Dake M. Zilver PTX Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease: Two Year Results, Presented at International Symposium on Endovascular Therapies (ISET), January 17, 2011, Miami Beach, Florida
4National Institute of Health, National Heart Lung and Blood Institute. How Is Peripheral Arterial Disease Treated?http://www.nhlbi.nih.gov/health/health-topics/topics/pad/treatment.html. Accessed August 10, 2012
Bloomington, Ind. — New recommendations from the Association of Clinical Researchers and Educators (ACRE) to guide relationships between physicians and industry could pave the way for a new era of medical advances and bring waves of breakthrough devices to patients worldwide.
Cook Medical on Thursday welcomed the efforts by ACRE to address interactions between physicians and industry and do it in a manner that does not build walls but instead leads to collaborations that advance medicine and education.
“Egregious misconduct by a few inappropriately colors the reputation of each and every health care stakeholder, whether they work in industry, in administration or as doctors,” said Bruce Gingles, vice president, global technology assessment and health policy at Cook Medical. “ACRE should be applauded for creating this fair and complete set of guidelines.”
“Egregious misconduct by a few inappropriately colors the reputation of each and every health care stakeholder, whether they work in industry, in administration or as doctors”
ACRE’s guidelines support collaborations between physicians and industry with recommendations that encourage rather than discourage innovation and education, with the ultimate goal of benefitting patients.
The guidelines define what constitutes “fair value” for receiving compensation and address areas of greater scrutiny that physicians should be aware of, such as formulary committees and institutional review boards. ACRE also offers recommendations on how physicians should disclose working relationships and calls for a formal rejection of the term“conflict of interest.”
The guidelines were co-authored by ACRE member J. Michael Gonzalez-Campoy, MD, PhD, FACE. Gonzalez-Campoy said rules must not get in the way of physicians who want industry input for breakthrough products and treatments.
“These guidelines achieve a much more reasonable approach to managing relationships so that physicians can actively participate in innovation and discovery of new treatments to enhance patient care and outcomes,” Gonzalez-Campoy said.
The universal model for medical innovation to enhance patient health and foster basic research stems from grants funded by industry and government to physicians and scientists. That funding in turn leads to published research, career advancement hile delivering patient care. Society benefits with medical advances and a stable academic health system.
In recent years a new model for innovation has been developed: university-affiliated translational research that brings together physicians, clinicians, scientists and industry to develop breakthroughs and patents. Revenues are reinvested in the university. New cures and advanced medical care benefit patients and society.
Freedom for physicians and industry to work together is critical to both approaches, Gingles said. ACRE’s guidelines will be published in the November/December issue of Endocrine Practice. In addition, the ACRE guidelines are available for download at http://aace.metapress.com/content/k12021244705x03w/?genre=article&id=doi%3a10.4158%2fEP12163.CO
“Most new product ideas address gaps in care, and they almost always come from clinicians rather than from industry,”Gingles said. But when there are complex regulations that discourage physician and industry meetings, innovation and patients pay the price.
“It’s a lot easier to blame an entire industry for isolated ethical problems than to create the sort of pragmatic relationships that lead to better patient care,” Gingles said.
About Cook Medical
A global pioneer in medical breakthroughs, Cook Medical is committed to creating effective solutions that benefit millions of patients worldwide. Today, we combine medical devices, drugs, biologic grafts and cell therapies across more than 16,000 products serving more than 40 medical specialties. Founded in 1963 by a visionary who put patient needs and ethical business practices first, Cook is a family-owned company. For more information, visit www.cookmedical.com. Follow Cook Medical on Twitter and LinkedIn.
Bloomington, Ind. — The Grant Street Inn has opened a new 16-room addition. The new building was designed and constructed to meet Leadership in Energy and Environmental Design (LEED) certification requirements. The facility will be the fourth LEED-certified building in Bloomington, and the first privately owned LEED-certified building in town. Certification, which is based on energy usage, sustainability and indoor air quality, will be completed upon review of energy and water consumption.
The Grant Street Inn’s new addition was built to match the 1890s style of the original inn. It is a part of CFC’s larger effort toward sustainable living. The addition includes green elements such as energy-saving light fixtures, low-flow showers, solar panel water heaters and a system to capture rainwater and use it to irrigate the lawn and solar panel water heaters.
“Guests can enjoy the usual comforts of our bed and breakfast as we work to reduce our carbon footprint.”
“We are so excited about this addition to the Grant Street Inn,” said Jim Murphy, president of CFC Properties. “Guests can enjoy the usual comforts of our bed and breakfast as we work to reduce our carbon footprint.”
This summer, guests of the inn were given gift cards to the local farmer’s market and encouraged to bring a plant back to the inn to be planted in the guest garden. The items they brought back were incorporated into the dayâs meals. These efforts, and others designed to get people thinking about things they can do to help the environment, will continue.
About CFC Properties
For more than 35 years, CFC Properties has been a leader in the historic restoration, promotion and revitalization of downtown Bloomington, Indiana. This includes the city’s largest bed and breakfast, the Grant Street Inn. CFC also recently expanded to Canton, Illinois, hometown of former president and CEO of Cook Group, Bill Cook. For more information, visit www.cfcproperties.com. Follow The Grant Street Inn on Twitter and Facebook.
Poway, Calif. — K-Tube Technologies is now working with two companies that will provide sales support and technical knowledge to customers. This collaboration with Medical Engineering Resources (MER) in Europe and Medical Metals in the United States will help all three companies reach more clients.
K-Tube makes more miniature stainless steel tubing than any other company in the United States. It is a Cook Group company whose primary customers are medical device engineers, designers and manufacturers.
“We’ve been focused on how we can give our customers more support,” said Greg May, president of K-Tube Technologies. “We wanted more than technical expertise. We wanted partners that share our core values. Both Medical Metals and MER-Europe were the right fit.”
MER-Europe is bringing K-Tube products to customers for the first time in every country in the European Union. MER-Europe provides a technical link between the customer and K-Tube. This helps engineers in the medical device industry find the right process and material for their designs.
“We are very proud of this new relationship because K-Tube’s specialty is a great complement to our existing portfolio of products,” said Tim Noppert, president of MER. “They and other Cook companies are like a big family. They work together and listen to one another.”
Medical Metals will expand K-Tube’s presence in New England, New York, New Jersey, Pennsylvania, Maryland and Delaware. Medical Metals specializes in machining and welding of tubing components, and it is highly respected in the nitinol community.
“K-Tube is ready to grow, and Medical Metals specializes in identifying trends in the market that will support its efforts,”said Rich Gordon, president of Medical Metals. “We’re excited to grow this agreement into a long-term relationship that benefits us both.”
“K-Tube is ready to grow, and Medical Metals specializes in identifying trends in the market that will support its efforts”
About K-Tube Technologies
Founded in 1974, K-Tube developed Laserweld technology to bring lower cost needles to the medical device market. K-Tube is now the largest U.S.-based manufacturer of miniature stainless steel tubing. K-Tube provides its customers with custom manufacturing, online ordering and a collaborative R D process for engineered tubing. For more information, visitwww.k-tube.com.
Las Vegas, Nevada — Three-year data from the Zilver® PTX® Randomized Controlled Trial of Paclitaxel-Eluting Stents for Femoropopliteal Disease from Cook Medical demonstrate 70.7 percent primary patency in the superficial femoral artery (SFA) at 36 months for patients treated with the paclitaxel-eluting stent. This compares to 49.1 percent patency for patients with percutaneous transluminal angioplasty and provisional bare metal stent placement in the 479-patient study.
In addition, the paclitaxel coating was shown to reduce the restenosis rate by 53 percent in a head-to-head comparison of provisional paclitaxel-eluting versus bare metal stent placement.
Michael Dake, M.D., professor in the Department of Cardiothoracic Surgery at Stanford University Medical School and medical director of the Cath/Angio Laboratories at Stanford University Medical Center, Palo Alto, California, presented the study findings today at the Vascular InterVentional Advances (VIVA) 2012 conference in Las Vegas, Nevada.
“These data from the largest clinical study ever conducted on peripheral stenting clearly show a sustained drug effect for paclitaxel-eluting stents versus bare metal stents after three-years”
“These data from the largest clinical study ever conducted on peripheral stenting clearly show a sustained drug effect for paclitaxel-eluting stents versus bare metal stents after three-years,” said Rob Lyles, vice president and global leader of Cook Medical’ Peripheral Intervention clinical division. “We are proud to have pioneered this important technology.”
Zilver PTX is neither approved by U.S. Food and Drug Administration nor available for sale in the United States. Dr. Dake, the global principal investigator for the Zilver PTX trial, is a paid consultant to Cook Medical regarding the research and development of medical devices.
About Cook Medical
A global pioneer in medical breakthroughs, Cook Medical is committed to creating effective solutions that benefit millions of patients worldwide. Today, we combine medical devices, drugs, biologic grafts and cell therapies across more than 16,000 products serving more than 40 medical specialties. Founded in 1963 by a visionary who put patient needs and ethical business practices first, Cook is a family-owned company. For more information, visit www.cookmedical.com. Follow Cook Medical on Twitter and LinkedIn.
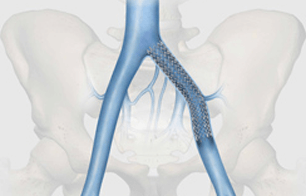
Bloomington, Ind. — Following Health Canada approval, Cook Medical made the Zilver Vena Venous Self-Expanding Stent available to physicians across Canada at the 2012 Annual Meeting of the Canadian Society for Vascular Surgery. Designed to restore blood flow in obstructed iliofemoral veins, Zilver Vena provides physicians with a tool designed specifically for stenting obstructed iliofemoral veins. This condition can arise for various reasons, including post-thrombotic syndrome in deep vein thrombosis (DVT) patients.
 According to a study by Dr. A. Rosales of the department of vascular surgery at Oslo University Hospital, post-thrombotic syndrome (PTS) characterized by reflux and/or obstruction can be expected to develop in up to 40 percent of DVT patients.1
According to a study by Dr. A. Rosales of the department of vascular surgery at Oslo University Hospital, post-thrombotic syndrome (PTS) characterized by reflux and/or obstruction can be expected to develop in up to 40 percent of DVT patients.1
Built on Cook’s established line of Zilver stents, the Zilver Vena stent is a flexible, self-expanding stent made with“shape memory” nitinol. Zilver Vena was developed to address a challenging clinical issue, the need to establish and maintain blood flow in obstructed iliofemoral veins.
The stent provides flexibility, consistent radial force, and continuous stent-to-vein wall apposition from end to end. Zilver Vena is currently available in 14 and 16 mm diameters and 60, 100 and 140 mm lengths to enable precise placement, and is compatible with 7 Fr sheaths and 9 Fr guiding catheters.
“As the only device of its kind available in Canada, Zilver Vena gives physicians a new treatment option for stenting diseased veins”
“As the only device of its kind available in Canada, Zilver Vena gives physicians a new treatment option for stenting diseased veins,” said Rob Lyles, vice president and global leader of Cook Medical’s Peripheral Intervention clinical division.
The Zilver Vena stent is currently under regulatory review by the U.S. Food and Drug Administration and is not approved for sale in the United States.
About Cook Medical
A global pioneer in medical breakthroughs, Cook Medical is committed to creating effective solutions that benefit millions of patients worldwide. Today, we combine medical devices, drugs, biologic grafts and cell therapies across more than 16,000 products serving more than 40 medical specialties. Founded in 1963 by a visionary who put patient needs and ethical business practices first, Cook is a family-owned company. For more information, visit www.cookmedical.com. Follow Cook Medical on Twitter and LinkedIn.
1 Rosales A. Sandbaek G, Jørgensen JJ. Stenting for chronic post-thrombotic vena cava and iliofemoral venous occlusions: mid-term patency and clinical outcome. Eur J Vasc Endovasc Surg.2010;40(2):234-240.

 According to a study by Dr. A. Rosales of the department of vascular surgery at Oslo University Hospital, post-thrombotic syndrome (PTS) characterized by reflux and/or obstruction can be expected to develop in up to 40 percent of DVT patients.1
According to a study by Dr. A. Rosales of the department of vascular surgery at Oslo University Hospital, post-thrombotic syndrome (PTS) characterized by reflux and/or obstruction can be expected to develop in up to 40 percent of DVT patients.1