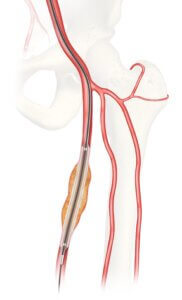
CAUTION: Investigational Device. Limited by United States law to investigational use.
Bloomington, Ind. — The U.S. Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an Investigational Device Exemption (IDE) study on the Advance Evero™ 18 Everolimus-Coated PTA Balloon Catheter (Evero DCB).
The clinical study will assess the safety and effectiveness of Evero DCB when compared to commercially available paclitaxel-coated balloons for the treatment of peripheral artery disease (PAD). Currently there are no everolimus-coated balloon devices commercially available in the US, and this IDE study is the first U.S. head-to-head evaluation of everolimus- and paclitaxel-coated balloons to treat lesions of the superficial femoral and popliteal arteries.
The EVERO Trial is a prospective, U.S. multi-center, stratified, blinded, randomized control trial with a parallel pharmacokinetic (PK) study. Cook intends to enroll 410 patients in the pivotal trial and 30 patients in the PK evaluation. The primary safety endpoint is a composite of freedom from device- or procedure-related death at 30 days, freedom from target limb major amputation at 12 months, and from target lesion revascularization (TLR) at 12 months. The primary effectiveness endpoint is primary patency, defined as peak systolic velocity ratio (PSVR) ≤2.4 at 12 months and freedom from clinically driven TLR (CD-TLR) at 12 months.
“Advance Evero 18 leverages our deep understanding and history of innovation in drug elution therapies for peripheral arterial disease,” said Dr. John Kaufman, Cook Medical’s chief medical officer. “The head-to-head study design will provide the answers that physicians and patients want. I’m confident that Advance Evero will be an excellent complement to our dedicated PAD portfolio aimed at improving long-term clinical outcomes for these patients.”
Additional information about the clinical study will be available at www.clinicaltrials.gov. You can also learn more about Cook’s portfolio of peripheral intervention products.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.
News coverage of this announcement
Endovascular Today: Cook’s Advance Evero 18 Everolimus-Coated Balloon IDE Study Plan Approved by FDA
Vascular News: FDA grants approval for IDE study on Advance Evero 18 everolimus-coated balloon catheter