Cook Medical has announced a new partnership with Mixxer Community Makerspace to bring more opportunities, more creativity, and more “I-can’t-believe-I-built-this” moments for people across the Triad.
 Through the new partnership, Cook will provide the resources to welcome even more creators to the Mixxer space. The collaboration will expand the lineup of affordable workshops—from woodworking and welding to 3D printing, electronics, textiles, and screen printing—each paired with expert, hands-on guidance.
Through the new partnership, Cook will provide the resources to welcome even more creators to the Mixxer space. The collaboration will expand the lineup of affordable workshops—from woodworking and welding to 3D printing, electronics, textiles, and screen printing—each paired with expert, hands-on guidance.
“Since our founding, we’ve been driven by invention and strengthened by connection,” said Tamisha Clark, vice president and general manager at Cook Medical’s Winston-Salem facility. “We believe innovation flourishes when everyone has a seat at the workbench, and that’s exactly what the Mixxer Community Makerspace does.”
“We’re turning ‘I built this!’ moments into real, career-ready skills—right here in the Triad—thanks to Cook Medical,” explained Elaine Lamson, executive director of Mixxer Community Makerspace. “Their support has made programs like Robotics and our first-ever Statewide Robotics Battle possible. By creating a space where participants can design, build, program, and battle robots, we’re filling a critical local gap and teaching skills in design, fabrication, electrical engineering, programming, and more—all in a fun, hands-on way. With Cook’s help, we’re empowering the next generation of innovators to build, create, and lead—right here at home.”
Why this partnership matters:
Hands-on classes at Mixxer give participants real-world experience in design, fabrication, and problem-solving, building practical skills that stick with them for life. Because workshops are intentionally affordable, the partnership turns equitable access into action, offering top-tier tools and training to anyone with an idea and the curiosity to chase it. By nurturing creativity and entrepreneurship, Mixxer strengthens Winston-Salem’s talent pipeline, fuels workforce readiness, and ultimately drives local economic growth.
How to jump in:
You can become a member, drop in for a class, volunteer your time or expertise, or simply spread the word. Community members can explore all the options at wsmixxer.org.
 Cook Medical and Siemens Healthineers today announced a strategic commercial partnership aimed at setting a new standard for interventional medicine. The collaboration combines the power of Siemens Healthineers real-time magnetic resonance imaging with Cook Medical’s deep interventional procedure expertise, along with new medical devices developed by Cook specifically for the MRI environment. Together, the companies are creating the Interventional MRI (iMRI) Suite1, the first integrated, ionizing radiation-free solution intended to transform how clinicians diagnose, plan and perform minimally invasive procedures.
Cook Medical and Siemens Healthineers today announced a strategic commercial partnership aimed at setting a new standard for interventional medicine. The collaboration combines the power of Siemens Healthineers real-time magnetic resonance imaging with Cook Medical’s deep interventional procedure expertise, along with new medical devices developed by Cook specifically for the MRI environment. Together, the companies are creating the Interventional MRI (iMRI) Suite1, the first integrated, ionizing radiation-free solution intended to transform how clinicians diagnose, plan and perform minimally invasive procedures.
“MRI in the interventional suite has always been a concept with tremendous clinical potential, but it has also faced significant barriers. At Siemens Healthineers, we are committed to breaking those barriers to unlock new clinical opportunities and advance patient care. Together with Cook Medical, we’re leading the way into a new era where iMRI becomes a precise radiation-free standard of care,” said Andreas Schneck, head of Magnetic Resonance at Siemens Healthineers.
By leveraging MRI’s unparalleled soft tissue contrast, iMRI enables precise guidance for minimally invasive interventions, particularly in areas such as interventional oncology for soft tissue tumors, pediatrics, and structural heart procedures, where accuracy and safety are paramount. This approach allows clinicians to navigate complex procedures with confidence while eliminating exposure to ionizing radiation for both patients and healthcare teams.
This first-of-its-kind collaboration delivers a turn-key solution designed to accelerate iMRI adoption and advance clinical impact in interventional radiology. It encompasses suite planning, an MRI scanner designed for interventions1, MRI-specific medical devices, specialized training, and ongoing clinical support. Cook Medical brings extensive procedural knowledge, innovative device design, and comprehensive educational programs, while Siemens Healthineers contributes its world-class MR imaging technologies and services, including dedicated interventional planning software.
The integrated iMRI suite reflects a shared commitment to innovation and collaboration, offering clinicians a complete framework, from conceptual suite design to staff training, to enable the adoption of this transformative technology for enhanced patient care.
“Our partnership with Siemens Healthineers is about more than device integration; it’s about revolutionizing interventional procedures,” said Peter Polverini, vice president of Cook Medical’s iMRI Division. “By merging the top-tier MRI systems of Siemens Healthineers with Cook’s advanced devices and expertise, we’re unlocking high-precision treatments that elevate patient care and empower clinicians.”
1 The product is currently under development and not available for sale in the U.S.A. Its future availability cannot be guaranteed.
Disclaimer: The product names and/or brands referred to are the property of their respective trademark holders.
For more information about Cook Medical’s iMRI division, visit CookMedical.com/divisions/iMRI and follow the journey on iMRI LinkedIn.
Further information on the iMRI suite can be found here.
Media contacts
Cook Medical
Marsha Lovejoy
+1 812 320 6903; marsha.lovejoy@cookmedical.com
Siemens Healthineers
Stefanie Haug
+49 173 635 8240; stefanie.haug@siemens-healthineers.com
Visit the Siemens Healthineers Press Center.
Subscribe to our “Medtech matters” newsletter on LinkedIn.

L-R: Rick Simms (Cook Medical, National Manager, GPO Account Executives), Amber Pastorek (Cook Medical, Account Executive Manager), Glenn Coleman (Premier, Chief Financial Officer & Chief Administrative Officer), Bob Stanley (Cook Medical, National GPO Account Executive), Brian Majoy (Cook Manager, Account Executive Manager), Chad Wissner (Cook Medial, Account Executive), Bruce Radcliff (Premier, President of Supply Chain Services) and Michael Alkire (Premier, President and CEO)
Bloomington, Ind. — Cook Medical is honored to receive a 2025 Legacy Supplier Award from Premier, Inc. This supplier award recognizes Cook’s deep connections and commitments to hospitals, supply chain leaders and other healthcare organizations.
Premier is a leading healthcare improvement and technology company that unites an alliance of more than 4,350 U.S. hospitals and health systems. Each year, Premier announces award winners at their Breakthroughs Conference. The awards in various categories go to organizations that demonstrate unwavering commitment to delivering top value products and services designed to continually improve patient care.
Cook Medical was honored to be among a select number of organizations that received the 2025 Legacy Supplier Award. Companies earn this award for long-standing support of Premier members through exceptional local customer service and engagement, value creation through clinical excellence and commitment to lower costs. Supplier Legacy Award winners have a tenure of more than three years as a Premier contracted supplier.
What separates Cook from other suppliers is our Customer Portal, which makes the ordering experience more convenient and efficient for customers. Cook also offers a supplier risk dashboard and a biweekly global supply chain report.
“For more than two decades, Cook Medical has been proud to collaborate with Premier,” said Bob Stanley, a national GPO account executive with Cook’s BusinessCare Integration team.”We are truly honored to receive this award from Premier. It reflects our shared mission and our dedication to delivering value to Premier’s membership. We’re excited about what lies ahead and continuing to grow with our customer’s evolving needs.”
To learn more about the advantages of partnering with Cook Medical, visit our BusinessCare Integration site.
About Premier, Inc.
Premier is a leading technology-driven healthcare improvement company, providing solutions to two-thirds of all healthcare providers in the U.S. Playing a critical role in the rapidly evolving healthcare industry, Premier unites providers, suppliers, payers and policymakers to make healthcare better with national scale, smarter with actionable intelligence and faster with novel technologies. With integrated data and analytics, collaboratives, supply chain solutions, consulting and other services, Premier enables better care and outcomes at a lower cost.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.

Some Cook Urology products that are included in the contract with Vizient
Bloomington, Ind. — Cook Medical has been awarded a contract from Vizient for our portfolio of urology devices. This agreement provides increased access to Cook’s urology products for Vizient clients while further strengthening Cook’s decades-long, physician-focused connections with hospitals across the country. Vizient is the nation’s largest provider-driven healthcare performance improvement company.
Through the contract, Vizient clients will experience streamlined ordering and delivery process and continue to receive excellent clinical and supply chain support from Cook Medical representatives. This agreement also allows physicians to continue treating patients with Cook products they know and trust at competitive price points and reliable product availability.
The urology products in this contract include:
“This contract is another milestone in our collaborative relationship,” said Rob Faulkner, senior director of global sales for Cook Medical’s MedSurg division. “We are excited to continue working with Vizient and making our comprehensive line of urological disposables more accessible to physicians and patients.”
In addition to this contract, Cook has previously earned recognition from Vizient. In 2023, Cook earned Vizient Supply Assurance Supplier of the Year Award. To learn more about the advantages of partnering with Cook Medical, visit our BusinessCare Integration site.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.
Cook Medical, a global medical device manufacturer, has announced a new collaboration to incorporate DuPont™ Tyvek® with Renewable Attribution into its medical device packaging. Together with Nelipak® Healthcare Packaging, an Authorized Converter of Tyvek® Healthcare Packaging products, Cook is taking action to deliver more sustainable product packaging that can be seamlessly integrated into existing healthcare packaging production processes.
The project involves the use of Tyvek® with Renewable Attribution in the packaging of Cook Medical’s Compass BDS® Biliary Plastic Stent and Resonance® Metallic Ureteral Stent, helping enable more sustainable healthcare packaging by reducing product carbon footprint and dependence on fossil fuels.
“We are committed to making sustainable choices across our business,” said Barry Slowey, chief sustainability officer at Cook Medical. “By integrating Tyvek® with Renewable Attribution into our packaging, we are taking meaningful steps toward reducing our carbon footprint and promoting environmentally responsible practices. We are excited to be an early adopter of this innovative offering, and we look forward to bringing it to our customers.”
“Nelipak® has long been an Authorized Converter of Tyvek®, delivering innovative healthcare packaging solutions worldwide. By including Tyvek® with Renewable Attribution, supplied from our ISCC PLUS certified sites, in our Ecovate® sustainable product portfolio, we are taking an important step to help our medical device customers achieve their carbon footprint reduction goals,” said Pat Chambliss, CEO at Nelipak®.
DuPont™ Tyvek® with Renewable Attribution has significantly lower CO2 emissions compared to conventional Tyvek®, while retaining its trusted performance and quality. The carbon footprint reduction of Tyvek® with Renewable Attribution is enabled by the partial replacement of fossil fuel materials with certified renewable sources.
This is achieved through the mass balance approach, in accordance with the International Sustainability and Carbon Certification (ISCC PLUS) system. The use of certified bio-circular feedstock attributed to Tyvek® enables lower CO2 emissions and helps to reduce the use of fossil fuels.
“To protect our planet, we need to be innovative, passionate, and most importantly, collaborative. We are excited to work with like-minded, industry-inspiring organizations, such as Cook Medical and Nelipak®, who are helping to lead the way toward the use of more sustainable solutions in healthcare packaging,” said David Domnisch, vice president and general manager, DuPont Tyvek®.
CAUTION – INVESTIGATIONAL DEVICE. Limited by United States law to investigational use.
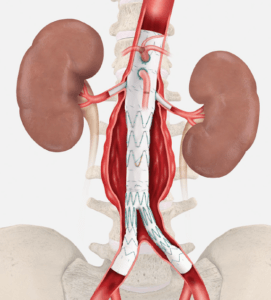
Cook Medical’s investigational ZFEN+ device
Bloomington, Ind. — Cook Medical has enrolled the final patient in the global clinical study of the ZENITH® FENESTRATED+ Endovascular Graft (ZFEN+). This milestone signifies the completion of patient recruitment in the pivotal study and demonstrates Cook’s commitment to innovating medical devices for the treatment of aortic diseases.
The investigational ZFEN+ clinical study is being conducted under an Investigational Device Exemption (IDE) approved by the U.S. Food and Drug Administration (FDA) and with authorization for a clinical investigation under Medicines and Healthcare products Regulatory Agency (MHRA). This clinical study will assess the safety and effectiveness of the ZFEN+ used in combination with the investigational Zenith® Universal Distal Body 2.0 Graft (Unibody2), the investigational Bentley BeGraft Balloon-Expandable FEVAR Bridging Stent Graft System (BeGraft) and the commercially available Zenith® Spiral-Z® AAA Iliac Leg Graft (ZSLE).
“Now that enrollment is complete, we are one step closer to understanding the outcomes of patients treated with ZFEN+ and, ultimately, to advancing care for patients with complex abdominal and thoracoabdominal aneurysms,” said Johnny LeBlanc, director, product management, Aortic at Cook Medical. “Reaching this milestone is a direct reflection of the dedication shown by our investigator partners, study coordinators, and—most importantly—the patients who supported the study with their time and trust. We look forward to analyzing the data and sharing the results with the clinical community.”
The ZFEN+ is predicated on the commercially available Zenith Fenestrated (ZFEN) AAA Endovascular Graft but extends the proximal margin of aneurysmal disease that can be treated endovascularly to include patients with more complex aortic disease involving one or more of the major visceral arteries. The ZFEN+ is an endovascular graft which includes up to 5 precisely located fenestrations, or a combination not to exceed a total of 5 made up of fenestrations and 1 scallop (cut-outs from the proximal margin of the endograft material) to accommodate visceral vessels. Physicians can order fenestrations and scallops specifically to match the patient’s unique anatomy. Overall, the ZFEN+ allows for the endovascular treatment of patients with aortic aneurysms and maximizes the seal zone to exclude the aneurysm. The product was granted Breakthrough Designation from the FDA in 2021.
“Reaching full enrollment in this important clinical study is a major milestone for Bentley and a significant step toward making our BeGraft Bridging stent available to patients in the United States. It underscores the strength of our collaboration with Cook Medical and reflects our shared commitment to advancing innovative vascular solutions that improve patient outcomes,” said Sebastian Büchert, Bentley CEO.
“Amazing work from the clinical research team on the enrollment of the final ZFEN+ patient. The clock is now ticking for us to have a patient specific solution for complex aortic aneurysms that will optimize seal without compromise,” said Dr. Gustavo Oderich, the global principal investigator of the clinical study.
Additional information about the clinical study will be available at www.clinicaltrials.gov.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.
CAUTION: Investigational Device. Limited by United States law to investigational use.
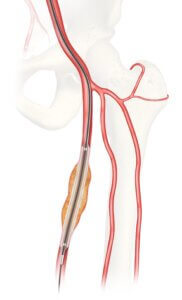
Cook Medical’s investigational Advance Evero™ 18 Everolimus-Coated PTA Balloon Catheter (Evero DCB)
Bloomington, Ind. — The U.S. Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an Investigational Device Exemption (IDE) study on the Advance Evero™ 18 Everolimus-Coated PTA Balloon Catheter (Evero DCB).
The clinical study will assess the safety and effectiveness of Evero DCB when compared to commercially available paclitaxel-coated balloons for the treatment of peripheral artery disease (PAD). Currently there are no everolimus-coated balloon devices commercially available in the US, and this IDE study is the first U.S. head-to-head evaluation of everolimus- and paclitaxel-coated balloons to treat lesions of the superficial femoral and popliteal arteries.
The EVERO Trial is a prospective, U.S. multi-center, stratified, blinded, randomized control trial with a parallel pharmacokinetic (PK) study. Cook intends to enroll 410 patients in the pivotal trial and 30 patients in the PK evaluation. The primary safety endpoint is a composite of freedom from device- or procedure-related death at 30 days, freedom from target limb major amputation at 12 months, and from target lesion revascularization (TLR) at 12 months. The primary effectiveness endpoint is primary patency, defined as peak systolic velocity ratio (PSVR) ≤2.4 at 12 months and freedom from clinically driven TLR (CD-TLR) at 12 months.
“Advance Evero 18 leverages our deep understanding and history of innovation in drug elution therapies for peripheral arterial disease,” said Dr. John Kaufman, Cook Medical’s chief medical officer. “The head-to-head study design will provide the answers that physicians and patients want. I’m confident that Advance Evero will be an excellent complement to our dedicated PAD portfolio aimed at improving long-term clinical outcomes for these patients.”
Additional information about the clinical study will be available at www.clinicaltrials.gov. You can also learn more about Cook’s portfolio of peripheral intervention products.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.
News coverage of this announcement
Endovascular Today: Cook’s Advance Evero 18 Everolimus-Coated Balloon IDE Study Plan Approved by FDA
Vascular News: FDA grants approval for IDE study on Advance Evero 18 everolimus-coated balloon catheter
 Bloomington, Ind. — Gayle Karch Cook, historical preservation activist and cofounder of the Cook companies alongside husband Bill Cook, passed away at 91 this morning. She is survived by her son Carl and daughter-in-law Marcy and their granddaughter, Eleanor.
Bloomington, Ind. — Gayle Karch Cook, historical preservation activist and cofounder of the Cook companies alongside husband Bill Cook, passed away at 91 this morning. She is survived by her son Carl and daughter-in-law Marcy and their granddaughter, Eleanor.
“Gayle’s spirit of innovation was evident in everything she did. Whatever she was involved in, she left it better than she found it. This was true of her philosophy for the ever-growing company, her extensive efforts in renovating historically significant places, and her daily interactions with other people. That’s her inspiring legacy that we will keep striving for,” said Pete Yonkman, president of Cook Medical and Cook Group.
In 1963, Bill and Gayle Cook created a small company named Cook Incorporated that manufactured three simple medical devices used to treat patients in less invasive ways than common surgical techniques of the time. Gayle was the company’s first quality control employee, meticulously checking the devices Bill made after he finished work each night.
That small company grew into what is today Cook Group, a family of ethical and entrepreneurial companies. Cook Group includes the flagship company, Cook Medical, a global company that invents, manufactures, and delivers devices for treating conditions in almost every system of the body. Cook Group also includes companies in the life sciences, property management, resort, and service businesses.

Bill, Carl and Gayle Cook
The family planned ahead for this event, and nothing will change for employees moving forward. Carl Cook, Gayle and Bill’s son, has been CEO of Cook Group since his father’s passing in 2011. Company leadership will remain the same with Pete Yonkman as president of Cook Group and Cook Medical and Steve Ferguson as Chairman of the Board.
Gayle Karch Cook, born March 1, 1934, in Evansville, Indiana, made excellence her goal in every aspect of her life. She was salutatorian of Bosse High School when she graduated in 1952, and she earned a Bachelor of Fine Arts from Indiana University in 1956. After graduating, she began her career in advertising in Chicago. After marrying her husband, Bill, they moved to Bloomington, Indiana. There, in the spare bedroom of their apartment, they officially began the company that would later become Cook Medical. The company’s focus has always been on the patient, with a goal to make surgeries minimally invasive.
 She was committed to creating a vibrant community, as she also served on the board of directors for the Star of Indiana Drum and Bugle Corps, the Bloomington Antique Mall, the Nature Conservancy, Indiana Chapter, and the board of trustees of the Indiana University Foundation.
She was committed to creating a vibrant community, as she also served on the board of directors for the Star of Indiana Drum and Bugle Corps, the Bloomington Antique Mall, the Nature Conservancy, Indiana Chapter, and the board of trustees of the Indiana University Foundation.
Gayle was an avid supporter of historic preservation, and she was heavily involved in many restoration projects. She spearheaded about 70 historic preservation and economic revitalization projects, including the much-lauded West Baden Springs and French Lick resorts; Indianapolis’ Central Avenue Methodist Church; and Beck’s Mill and Cedar Farm complex in southern Indiana. The first property she restored and refurnished was the Colonel William Jones House, built in 1835. The house, which is on the National Register of Historic Places, is now a museum open to the public as a State Historic Site. Gayle restored historic sites to their original state, including the James Cochran house, the Illinois Central Freight Depot, the 8-story Graham Hotel, and more than 17 structures in the central square of Bloomington, Indiana.
 In addition to her involvement in the restoration work itself, Gayle served on the boards of many restoration organizations. She was on the advisory committee of the Wylie House Museum, the Board of Governors of the Monroe County Historical Museum, the board of the Historic Landmarks Foundation of Indiana, and was an appointee of the State Historic Preservation Review Board. She was also a founder of the Monroe County History Center Annual Garage Sale which has become a staple in the Bloomington community. With every project, Gayle’s philosophy was to restore not just the site itself, but also the character and beauty of the location for present and future generations. Historic preservation wasn’t just good business; it was an investment in the community.
In addition to her involvement in the restoration work itself, Gayle served on the boards of many restoration organizations. She was on the advisory committee of the Wylie House Museum, the Board of Governors of the Monroe County Historical Museum, the board of the Historic Landmarks Foundation of Indiana, and was an appointee of the State Historic Preservation Review Board. She was also a founder of the Monroe County History Center Annual Garage Sale which has become a staple in the Bloomington community. With every project, Gayle’s philosophy was to restore not just the site itself, but also the character and beauty of the location for present and future generations. Historic preservation wasn’t just good business; it was an investment in the community.
Gayle authored and published two books detailing her passion for Indiana’s history. The first, A Guide to Southern Indiana, was published in 1972. The other, Monroe County in Focus: Portrait of an Indiana County, was published in 1990.
A Phi Beta Kappa graduate of IU, Gayle earned a Bachelor of Arts degree in fine arts in 1956. She and her late husband were generous, far-sighted benefactors of IU’s Jacobs School of Music, School of Education, School of Medicine and Kelley School of Business, as well as supporting IU Athletics, the Wylie House Museum, the Wells Scholars program and research in the College of Arts and Sciences.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.
 Through the new partnership, Cook will provide the resources to welcome even more creators to the Mixxer space. The collaboration will expand the lineup of affordable workshops—from woodworking and welding to 3D printing, electronics, textiles, and screen printing—each paired with expert, hands-on guidance.
Through the new partnership, Cook will provide the resources to welcome even more creators to the Mixxer space. The collaboration will expand the lineup of affordable workshops—from woodworking and welding to 3D printing, electronics, textiles, and screen printing—each paired with expert, hands-on guidance.








