
The COVID-19 pandemic has changed the way physicians are thinking about patient care, forcing them to adapt to new technologies and protocols. It has also given physicians the opportunity to think about the future of medicine, including what it may look like after COVID-19. Through this blog series, we’re interviewing physicians to share their first-hand experiences on how they’re adapting their practices during the COVID-19 pandemic, as well as their thoughts on the future of patient care.
 For this post we interviewed Dr. Brandon Isaacson, to get his perspective on patient care and what the protocols for otologic procedures have looked like during the COVID-19 pandemic. Dr. Isaacson is a professor in the Department of Otolaryngology—Head and Neck Surgery at UT Southwestern Medical Center and serves as co-director of UT Southwestern’s Comprehensive Skull Base Program. Below are some highlights of the interview.
For this post we interviewed Dr. Brandon Isaacson, to get his perspective on patient care and what the protocols for otologic procedures have looked like during the COVID-19 pandemic. Dr. Isaacson is a professor in the Department of Otolaryngology—Head and Neck Surgery at UT Southwestern Medical Center and serves as co-director of UT Southwestern’s Comprehensive Skull Base Program. Below are some highlights of the interview.
Cook: What impact has COVID-19 had on otologic procedures?
Dr. Isaacson: Initially, when the pandemic started in March 2020, all elective surgical procedures were placed on hold. Our facility resumed otologic surgical procedures in May 2020, testing all patients for COVID prior to surgery. N95 masks and appropriate PPE were provided for all staff in the operating room regardless of a negative preoperative COVID test. Surgical volumes were progressively increased from May through June until we were back at pre-pandemic levels, using the same preoperative COVID testing protocols and PPE requirements.
Appropriate PPE was worn in the clinic for all aerosol-generating procedures, but pre-procedure COVID testing was not performed. At present, we are still performing otologic procedures using the same testing and PPE protocols that were initiated at the beginning of the pandemic.
How are you currently prioritizing cases?
We have been at full OR capacity since early this year. We did have a brief slowdown at Children’s and the county hospital during the January COVID surge.
How have otologic procedures changed due to COVID-19 in your specific practice?
Clinic in-person visits are back to pre-pandemic levels. We have increased our use of telemedicine, which was significant at the beginning of the pandemic. At the beginning of the pandemic, I was seeing approximately 20 patients per week via telemedicine, which is now down to 4 to 6 telemedicine visits per week.
How are physical examinations being performed, if at all?
We have been performing relevant physical exams since we reinitiated in-person clinic visits in May 2020.
Is the room setup any different?
Vigorous sterilization procedures are performed in each exam room after each visit. If an aerosol-generating procedure (AGP) is performed, the room is closed afterwards.
How have you implemented PPE into your practice?
We have all been wearing face masks and using hand washing, gloves, and eye protection for each in-person encounter.
How are you screening patients for COVID-19?
Each outpatient is screened with a temperature check and a COVID-19 symptoms inventory at check-in.
The COVID-19 pandemic, which resulted in the postponement of elective procedures and has led to new COVID testing protocols and PPE requirements, has also caused otolaryngologists to take a closer look at their procedures in an effort to reduce exposure risks. Ayache et al. recently discussed the benefits of a minimally invasive endoscopic approach as an alternative to mastoidectomy and highlighted the benefits of transcanal endoscopic ear surgery (TEES) as a means to treat many otologic conditions, avoiding the risks associated with aerosol-generating procedures.1
If a virus like SARS-CoV-2 is present during a mastoidectomy, the viral particles may aerosolize and present an increased risk of transmission to the OR staff.1 Rather than making an incision behind the ear and drilling through bone to access the ear canal, TEES utilizes a rigid endoscope through the ear canal to access the middle ear, thus minimizing the risk of exposure.

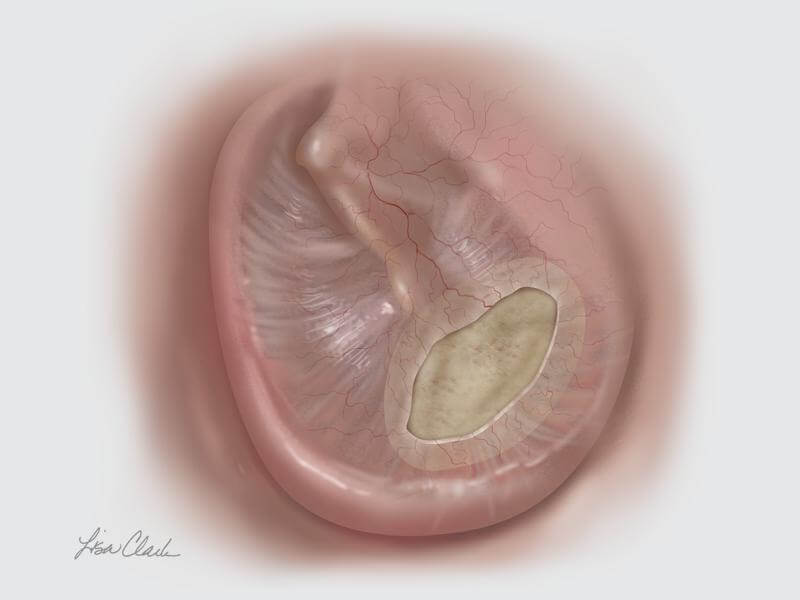 When faced with a tympanic membrane perforation, TEES can be used in conjunction with the Biodesign® Otologic Repair Graft to avoid the need for an external incision. The Biodesign Otologic Repair Graft is an off-the-shelf alternative to autologous tissue and, when paired with the endoscope, allows for a minimally invasive approach to tympanic membrane repair.
When faced with a tympanic membrane perforation, TEES can be used in conjunction with the Biodesign® Otologic Repair Graft to avoid the need for an external incision. The Biodesign Otologic Repair Graft is an off-the-shelf alternative to autologous tissue and, when paired with the endoscope, allows for a minimally invasive approach to tympanic membrane repair.
- Ayache S, Kutz W, Isaacson B, et al. COVID‑19 and ear endoscopy in otologic practices. Eur Arch Otorhinolaryngol. 2021;278(6):2133-2135.
Cook Medical recently announced that the Hercules® 100 Transnasal Esophageal Balloon is now commercially available to physicians in the US. This product is the first balloon designed specifically for transnasal esophageal procedures. With the Hercules 100, ear, nose, and throat (ENT) physicians have another tool available to help treat esophageal strictures. Read the full press release here.

COVID-19 has changed the way that physicians are thinking about patient care, forcing them to adapt to new technologies and protocols. It has also given physicians the opportunity to think about the future of medicine, including what it may look like after COVID-19. Through this blog series, we’re interviewing physicians to share their first-hand experiences on how they’re adapting their practices during the COVID-19 pandemic, as well as their thoughts on the future of patient care.
 We interviewed Rohan R. Walvekar, MD, to get his perspective on patient care and what the future of sialendoscopy procedures may look like during the COVID-19 pandemic. Dr. Walvekar is the Director of Salivary Endoscopy Service and the Co-Director of ENT Service University Medical Center in the department of Otolaryngology Head & Neck surgery at the Louisiana State University Health Sciences Center in New Orleans, Louisiana.
We interviewed Rohan R. Walvekar, MD, to get his perspective on patient care and what the future of sialendoscopy procedures may look like during the COVID-19 pandemic. Dr. Walvekar is the Director of Salivary Endoscopy Service and the Co-Director of ENT Service University Medical Center in the department of Otolaryngology Head & Neck surgery at the Louisiana State University Health Sciences Center in New Orleans, Louisiana.
Below are some highlights of the interview. For the full interview, download the PDF here.
The future of sialendoscopy procedures
How have sialendoscopy procedures changed to adapt to COVID-19 in your practice?
 COVID-19 has definitely changed our practice patterns, especially for outpatient services. Many of the otolaryngology procedures, including sialendoscopy, are now considered high-risk since they are aerosol-generating procedures (AGPs). Patients who need an interventional procedure, whether it is a routine flexible endoscopy as a normal part of a head and neck examination during their visit or an interventional sialendoscopy procedure, are now required to have a COVID-19 test within 48 to 72 hours of their in-office procedure, since these are all considered to be AGPs. Some of our clinic spaces have been re-structured to provide negative pressure ventilation in the rooms. In-office AGPs are performed in these negative pressure rooms with proper PPE precautions. Many practices at some sites, including ours, have moved to the use of disposable scopes and equipment when possible for COVID-19-positive patients. Social distancing and its impact on triaging patients, the need for COVID-19 testing, and the need to use additional sterilization procedures to clean and turnover clinic rooms, e.g., UV light technology, has significantly reduced overall patient volumes in clinics. Some of these factors have also impacted surgical turnovers in the hospital setting, impacting surgical volumes. However, these precautions have been vital to help keep our patients, staff, and other healthcare professionals safe during this pandemic.
COVID-19 has definitely changed our practice patterns, especially for outpatient services. Many of the otolaryngology procedures, including sialendoscopy, are now considered high-risk since they are aerosol-generating procedures (AGPs). Patients who need an interventional procedure, whether it is a routine flexible endoscopy as a normal part of a head and neck examination during their visit or an interventional sialendoscopy procedure, are now required to have a COVID-19 test within 48 to 72 hours of their in-office procedure, since these are all considered to be AGPs. Some of our clinic spaces have been re-structured to provide negative pressure ventilation in the rooms. In-office AGPs are performed in these negative pressure rooms with proper PPE precautions. Many practices at some sites, including ours, have moved to the use of disposable scopes and equipment when possible for COVID-19-positive patients. Social distancing and its impact on triaging patients, the need for COVID-19 testing, and the need to use additional sterilization procedures to clean and turnover clinic rooms, e.g., UV light technology, has significantly reduced overall patient volumes in clinics. Some of these factors have also impacted surgical turnovers in the hospital setting, impacting surgical volumes. However, these precautions have been vital to help keep our patients, staff, and other healthcare professionals safe during this pandemic.
How will the procedural landscape for salivary gland treatment change?
The thought process for salivary intervention will be influenced by the COVID-19 status. For COVID-19-negative patients, the procedural landscape may remain the same. However, if the patient is COVID-19 positive, then the surgical intervention will be postponed until the patient is past the infective phase, i.e., after 14 days of quarantine and after demonstrating two successive COVID-19-negative tests. Or, if intervention is necessary, a gland excision route may be preferred for certain indications where intra-oral intervention may be complex and have a high risk of viral shedding—for example, an intermediate sized (5-6 mm) hilar stone in the submandibular gland that needs a combined approach procedure, laser fragmentation of hilar-intraglandular stones, or possibly an endoscopic management of high-grade diffuse stenosis. All of these conditions are surgical challenges.
It is more likely that procedures will move from in office to the operating room setting as the intervention is more controlled and measured. All healthcare professionals can take adequate PPE precautions, and once the patient is intubated, the risk of viral shedding decreases compared to an awake patient, who may cough, sneeze, or have a robust gag reflex.
Innovations will come in various ways to help the current situation. Innovations such as the ACE2-X solution could be helpful, if proven effective, to help reduce viral burden and make intervention safer. There are many new innovations, such as innovative techniques to perform examinations, negative pressure environments, and perforated face masks or helmets to allow ENT examinations.
Sialendoscopy products

Do you anticipate an increase in demand in Cook’s minimally invasive sialendoscopy products?
I do anticipate an increase in the demand for certain Cook products, especially the disposable access catheters and wire guides. There also may be an increase demand for the use of the SialoCath® Salivary Duct Catheter, which may be considered for irrigation and washout procedures for chronic sialadenitis, radioactive iodine induced sialadenitis, and Sjogren’s syndrome. Dilation followed by only irrigation with saline, or antibiotics or steroids, or a combination thereof may be a less-invasive alternative to endoscopy and pose a reduced risk of contamination to the salivary endoscope. For centers equipped with negative pressure clinics, the ability to perform these procedures may help reduce the demand for operating room time, which is already reduced due to the requirement for resource management and PPE conservation.

In the full interview, Dr. Walvekar also answers the following questions:
The future of sialendoscopy procedures
- As otolaryngology procedures start back up, how quickly do you see sialendoscopy procedures returning?
- How have patient consultations and physical examinations changed?
- How have you implemented PPE into your practice?
- How are the examination rooms set up?
- How are you screening patients for COVID-19?
- We have heard of some physicians changing from betadine to chlorhexidine for prep prior to salivary and sialendoscopy procedures. Do you have any thoughts on this and the impact on COVID-19?
- How do you see hands-on educational courses adapting to further physician education?
- Will there be a shift away from surgical procedures?
Sialendoscopy products
- Do you anticipate an increased usage of the Advance® Salivary Duct Balloon Catheter by bringing more stricture patients into the office and using ultrasound?
- Do you anticipate an increase in the preference of disposable sialendoscopy devices over reusable devices?
To learn more about Cook’s products for sialendoscopy, click here.
Dr. Walvekar is a paid consultant of Cook Medical.
The opinions expressed by Dr. Walvekar in this interview are his own, and not the opinions of Cook Medical, and represent his experience within his practice.

What is Biodeisgn Tissue Repair Technology? Biodesign grafts are made from a platform technology behind numerous tissue-repair products that span multiple medical specialties. The biomaterial used for Otolaryngology Head and Neck Surgery (OHNS) products is small intestinal submucosa (SIS).SIS is a durable collagen material that is isolated from porcine intestine. When used for soft-tissue repair, SIS provides a scaffold for host cells. The OHNS division’s primary areas of focus for soft-tissue repair are sinonasal, otologic and dura.
Why SIS technology?
Often, physicians use a patient’s own tissue for OHNS procedures. Drawbacks to this approach include the time it takes to harvest patient tissue and additional incisions for the patient. Additionally, patient tissue can only be harvested from a donor site once. Pigs, on the other hand, are a readily available source for tissue extraction. The material from pigs has also been shown to be safe for medical devices with no known porcine transmissible spongiform encephalopathies (TSE). The submucosa within the small intestine also survives one of the harshest environments of the body and supports rapid cell turnover.
What is an extracellular matrix (ECM)?
The ECM is the structural substance that surrounds cells in nearly all tissues in the body. While the composition of the ECM varies by the type of tissue, it is generally composed of four major types of molecules.
- Structural proteins, such as collagen and elastin, that provide the main structural framework in ECM
- Glycoproteins that help bind cells to the collagen
- Glycosaminoglycans and proteoglycans that keep the matrix well hydrated by absorbing water and binding growth factors
This complex composition is retained during manufacturing using a unique process that confirms biocompatibility and leaves the extracellular matrix intact. Biodesign has been used in many applications throughout the body. For more information on our OHNS Biodesign product offerings, visit our product pages:
https://www.cookmedical.com/products/96f4b36f-75f7-4ebf-aef0-611faca117c8/
https://www.cookmedical.com/products/3165f1ce-66d0-4a7a-bf36-1584698e7174/
https://www.cookmedical.com/products/2cc93582-7339-4b59-82d1-d7347ce604a9/
https://www.cookmedical.com/products/sur_dur_webds/

 For this post we interviewed Dr. Brandon Isaacson, to get his perspective on patient care and what the protocols for otologic procedures have looked like during the COVID-19 pandemic. Dr. Isaacson is a professor in the Department of Otolaryngology—Head and Neck Surgery at UT Southwestern Medical Center and serves as co-director of UT Southwestern’s Comprehensive Skull Base Program. Below are some highlights of the interview.
For this post we interviewed Dr. Brandon Isaacson, to get his perspective on patient care and what the protocols for otologic procedures have looked like during the COVID-19 pandemic. Dr. Isaacson is a professor in the Department of Otolaryngology—Head and Neck Surgery at UT Southwestern Medical Center and serves as co-director of UT Southwestern’s Comprehensive Skull Base Program. Below are some highlights of the interview. When faced with a tympanic membrane perforation, TEES can be used in conjunction with the Biodesign® Otologic Repair Graft to avoid the need for an external incision. The Biodesign Otologic Repair Graft is an off-the-shelf alternative to autologous tissue and, when paired with the endoscope, allows for a minimally invasive approach to tympanic membrane repair.
When faced with a tympanic membrane perforation, TEES can be used in conjunction with the Biodesign® Otologic Repair Graft to avoid the need for an external incision. The Biodesign Otologic Repair Graft is an off-the-shelf alternative to autologous tissue and, when paired with the endoscope, allows for a minimally invasive approach to tympanic membrane repair.

 We interviewed
We interviewed  COVID-19 has definitely changed our practice patterns, especially for outpatient services. Many of the otolaryngology procedures, including sialendoscopy, are now considered high-risk since they are aerosol-generating procedures (AGPs). Patients who need an interventional procedure, whether it is a routine flexible endoscopy as a normal part of a head and neck examination during their visit or an interventional sialendoscopy procedure, are now required to have a COVID-19 test within 48 to 72 hours of their in-office procedure, since these are all considered to be AGPs. Some of our clinic spaces have been re-structured to provide negative pressure ventilation in the rooms. In-office AGPs are performed in these negative pressure rooms with proper PPE precautions. Many practices at some sites, including ours, have moved to the use of disposable scopes and equipment when possible for COVID-19-positive patients. Social distancing and its impact on triaging patients, the need for COVID-19 testing, and the need to use additional sterilization procedures to clean and turnover clinic rooms, e.g., UV light technology, has significantly reduced overall patient volumes in clinics. Some of these factors have also impacted surgical turnovers in the hospital setting, impacting surgical volumes. However, these precautions have been vital to help keep our patients, staff, and other healthcare professionals safe during this pandemic.
COVID-19 has definitely changed our practice patterns, especially for outpatient services. Many of the otolaryngology procedures, including sialendoscopy, are now considered high-risk since they are aerosol-generating procedures (AGPs). Patients who need an interventional procedure, whether it is a routine flexible endoscopy as a normal part of a head and neck examination during their visit or an interventional sialendoscopy procedure, are now required to have a COVID-19 test within 48 to 72 hours of their in-office procedure, since these are all considered to be AGPs. Some of our clinic spaces have been re-structured to provide negative pressure ventilation in the rooms. In-office AGPs are performed in these negative pressure rooms with proper PPE precautions. Many practices at some sites, including ours, have moved to the use of disposable scopes and equipment when possible for COVID-19-positive patients. Social distancing and its impact on triaging patients, the need for COVID-19 testing, and the need to use additional sterilization procedures to clean and turnover clinic rooms, e.g., UV light technology, has significantly reduced overall patient volumes in clinics. Some of these factors have also impacted surgical turnovers in the hospital setting, impacting surgical volumes. However, these precautions have been vital to help keep our patients, staff, and other healthcare professionals safe during this pandemic.

