Each year, approximately 900,000 people in the US are affected by pulmonary embolism (PE) and/or deep vein thrombosis, according to the American Lung Association.1 PE is a serious and potentially life-threatening condition that can result from blood clot formation in the legs, known as deep vein thrombosis (DVT). These clots can travel to the lungs and can lead to serious health consequences. Inferior vena cava (IVC) filters may be indicated for the prevention of recurrent PE when anticoagulant therapy is contraindicated; anticoagulant therapy has failed in thromboembolic diseases; emergency treatment following massive PE where anticipated benefits of conventional therapy are reduced; and chronic, recurrent PE where anticoagulant therapy has failed or is contraindicated.
Backed by clinical data: the CIVC study
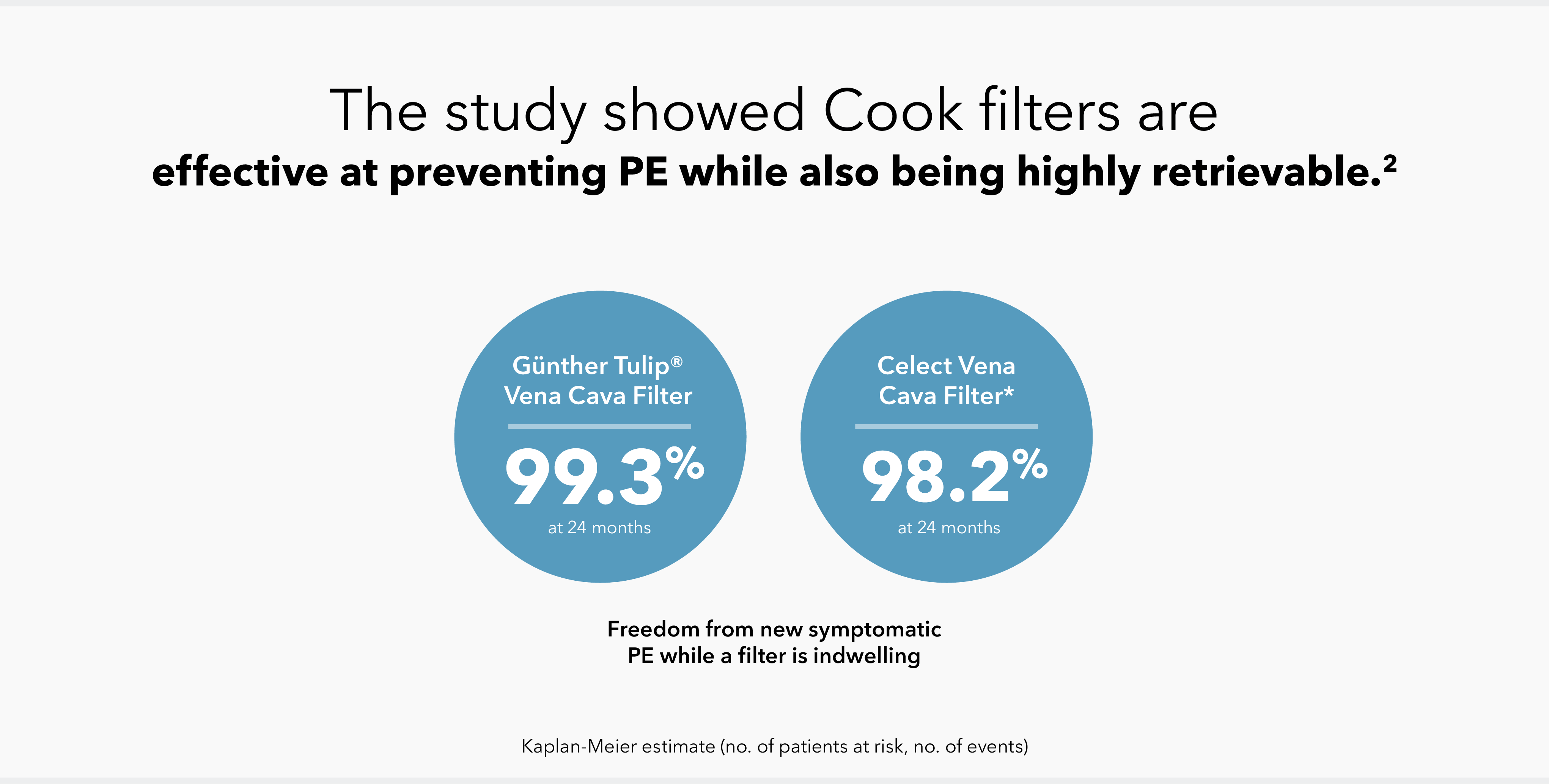
The findings from the Cook Inferior Vena Cava Filter (CIVC) study demonstrates technical success in placement and retrieval while maintaining safety and effectiveness outcomes.2
The study, which evaluated the Günther Tulip and Celect IVC filters, found that freedom from new symptomatic PE remained high, with low rates of major adverse events (MAEs) such as filter migration, fracture, and embolization.2 Notably, retrieval was attempted in over 70% of patients, with a 94.9% success rate for the overall patient cohort,2 demonstrating the utility of IVC filters in clinical practice when placed and retrieved according to the Instructions for Use and the Society for Interventional Radiology guidelines.3
Study background
The CIVC study was a 2-year, prospective, multicenter, single-arm, investigational-device-exemption (IDE) study.2
From 2014 to 2017, investigators studied clinical results in patients who received a Cook IVC filter—either a Günther Tulip Vena Cava Filter or a Celect Vena Cava Filter.2 The study’s primary endpoints were technical placement success and 1-year freedom from new symptomatic PE while a filter was indwelling, as well as 1-year freedom from MAEs.2 Follow-up was conducted for 2 years or for 30 days after filter retrieval.2
Primary effectiveness endpoint:
Rate of technical placement success and 12-month freedom from new symptomatic PE while a filter was indwelling2
Primary safety endpoint:
Rate of 12-month freedom from MAEs (clinical perforation, clinical migration, clinical fracture, embolization of the filter or filter fragments to the heart or lungs, IVC thrombotic occlusion, new symptomatic DVT while the filter was indwelling, access site complications with clinical sequelae, procedure- or device-related death)2
Secondary endpoints:
Rate of technical placement success and 12-month freedom from new symptomatic PE while a filter was indwelling; rate of 12-month freedom from MAEs; rate of 12-month freedom from grade 2 or grade 3 filter leg interaction with the IVC; filter migration; filter fracture; and filter embolization (assessed for the overall cohort and for each stratum)2
Secondary measures:
PE, DVT (symptomatic and total), IVC thrombotic occlusion, and postplacement filter outcomes including fracture, embolization, significant migration, filter leg interaction with the IVC wall, clinical perforation, filter retrieval measures, access site adverse events with clinical sequelae, and procedure- or device-related death (assessed for the overall cohort and for each stratum)2
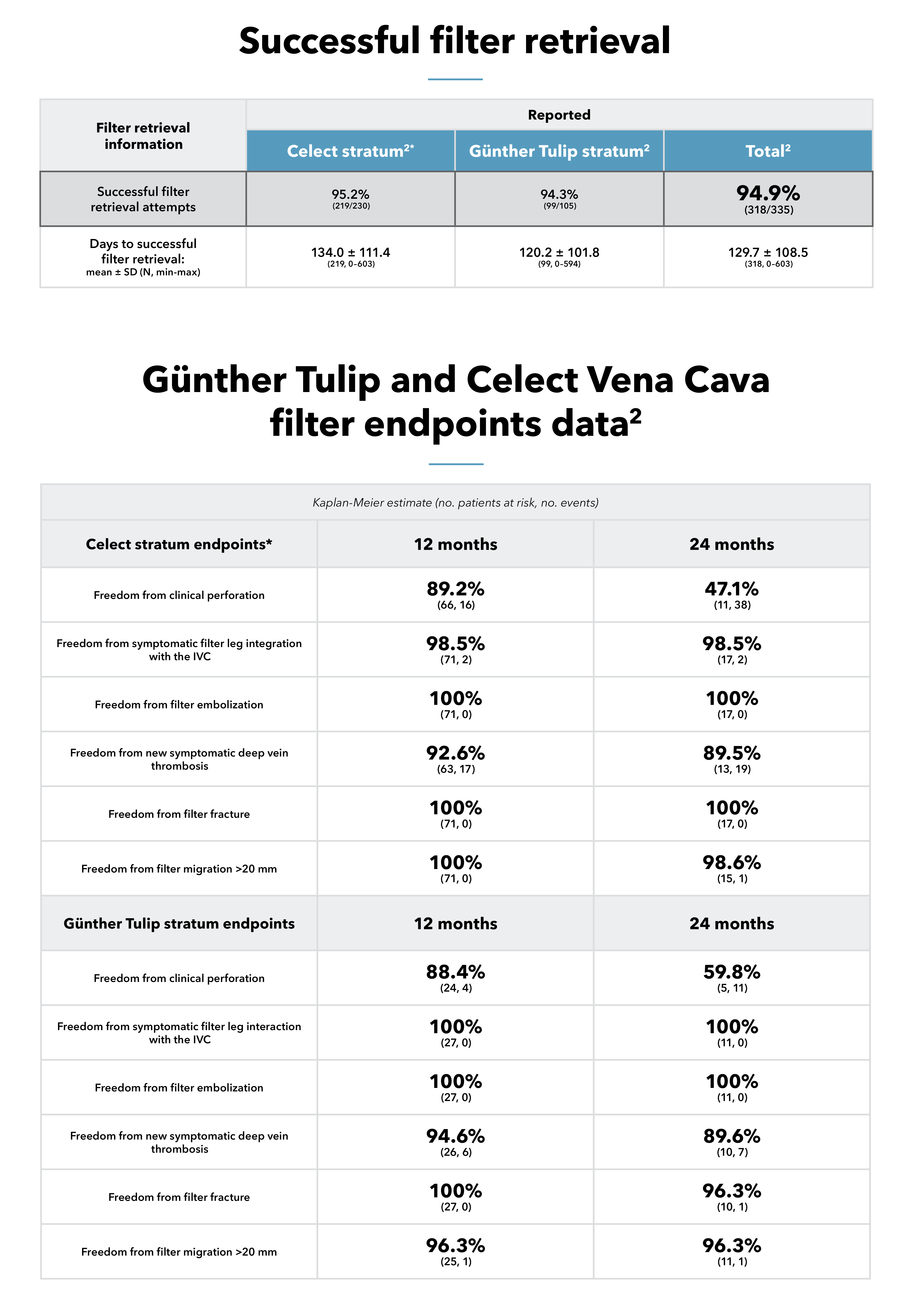
*Celect stratum consisted of Celect and Celect Platinum vena cava filters.
thrombus in the deep veins, usually of the lower extremities or pelvis; documented by contrast venography, duplex ultrasound CT, or MR venography
postplacement movement of the filter or its components to a distant anatomical site completely out of the target zone (e.g., heart or lungs); documented by imaging or at autopsy
any loss of structural integrity (breakage or separation) of the filter; documented by imaging or at autopsy
change in filter position compared with its deployed position (cranial or caudal); classified as significant (>20 mm) or insignificant (<20 mm); documented by plain radiography, CT, or venography
Emboli to lungs via the pulmonary artery, which can arise from DVT in the lower extremities or pelvis; documented using pulmonary arteriography, cross-sectional imaging, significant change in ventilation or perfusion lung scan, or at autopsy
deployment of a filter in a location suitable to provide sufficient mechanical protection against PE with no filter deformation, fracture, premature release, or clinical migration
Caution: Federal (US) law restricts the use of this device to sale by or on the order of a physician.
Please refer to the product’s Instructions for Use (IFU) for full prescribing information, warnings, precautions, contraindications, and potential adverse events.