Deep venous obstruction
Data shows Zilver Vena® benefits patients through three years
The VIVO Clinical Study represents the first Investigational Device Exemption for a venous stent and enrolled 243 patients considered representative of a real-world population with iliofemoral venous outflow obstruction. The study results met the 1-year endpoints, and the 3-year results support the continued safety and effectiveness of the stent.1,2
Main highlights of the study’s three-year data include:
- Patients treated in the VIVO-US study are reflective of a real-world population, including clinical presentations of 105 PTS (43.2%), 79 NIVL (33%), and 59 acute DVT (24%) patients.
- 32.5% of patients had stent extension below the inguinal ligament into the common femoral and/or femoral vein./li>
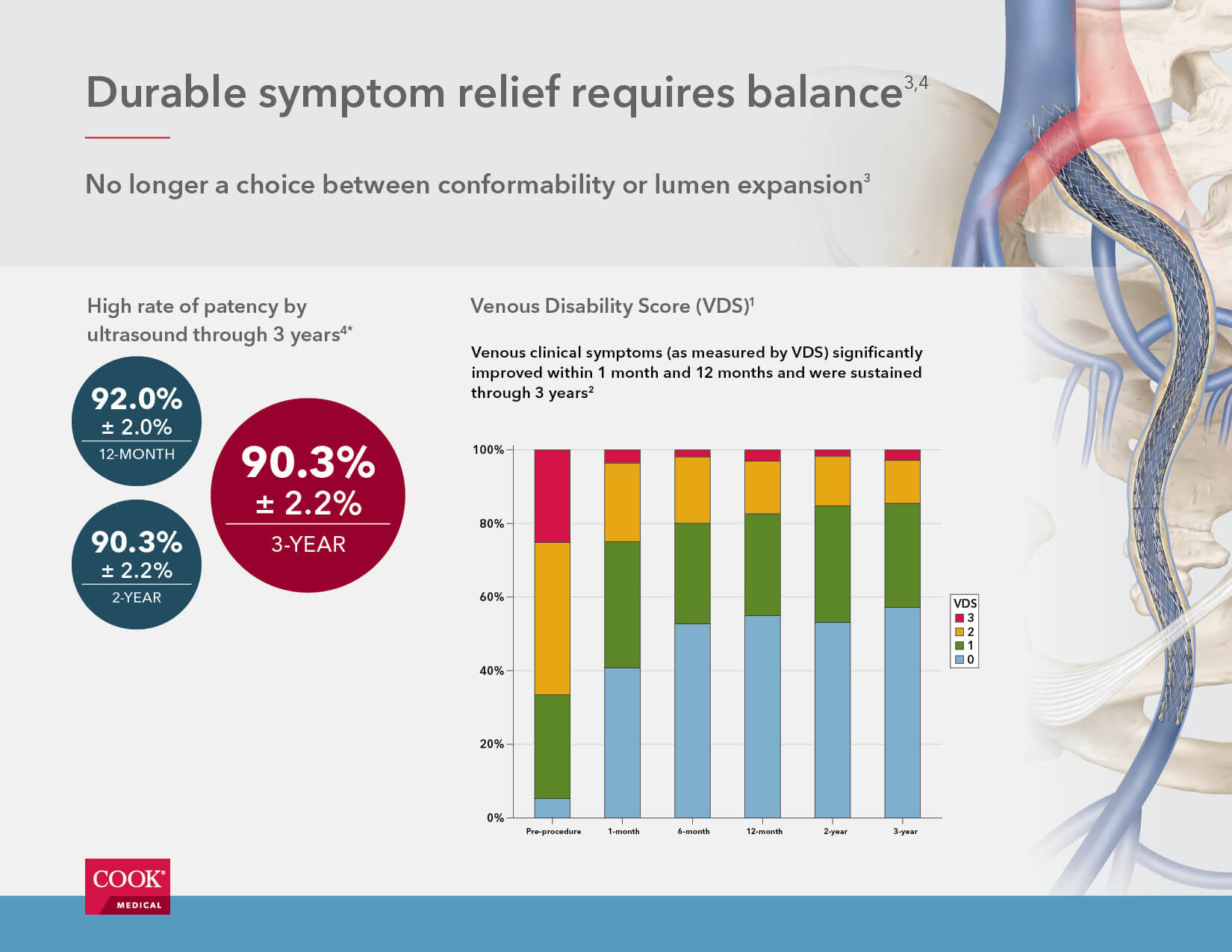
- Zilver Vena is associated with a high rate of patency by ultrasound (90.3% ± 2.2%) three years after the initial procedure.
- Patients treated with Zilver Vena demonstrated significant improvement in clinical symptoms as measured by VCSS (venous clinical severity score), VDS (venous disability score), CEAP “C” classification (clinical condition), and CIVIQ (chronic venous insufficiency quality of life). These measures improved significantly (p<0.0001) by 1 month post-procedure, with continued or sustained improvement through 3 years.
- Stent integrity was maintained through 3 years, with no fractures identified by the core laboratory.
Read the FDA Summary of Safety & Effectiveness
PE prevention
Published Societal Guidelines for IVC filter placement
These three guidelines are among the most widely referenced and share the following indications for IVC filter placement:
- Complication of anticoagulation
- Contraindication to anticoagulation
- Failure of anticoagulation
- Inability to achieve adequate anticoagulation
- Massive PE with residual DVT in a patient at risk for further PE
- Recurrent PE despite adequate therapy
CIRSE5
Patients with evidence of pulmonary embolus or IVC, iliac, or femoral-popliteal DVT and one or more of the following:
- Contraindication to anticoagulation
- Complication of anticoagulation
- Failure of anticoagulation
- i. recurrent PE despite adequate therapy
- ii. inability to achieve adequate anticoagulation
Additional indications for selected patients:
- Massive pulmonary embolism with residual deep venous thrombus in a patient at risk of further PE
- Free floating iliofemoral or inferior vena cava thrombus
- Severe cardiopulmonary disease and DVT (e.g. cor pulmonale with pulmonary hypertension)
- Poor compliance with anticoagulant medications
- Severe trauma without documented PE or DVT
- Closed head injury
- Spinal cord injury
- Multiple long bone or pelvic fractures
- High risk patients (e.g. immobilised, ICU patients, prophylactic pre-operative placement in patients with multiple risk factors of venous thromboembolism)
SIR6
Patients with evidence of PE or IVC, iliac, or femoral-popliteal DVT and one or more of the following:
- Absolute or relative contraindication to anticoagulation
- Complication of anticoagulation
- Failure of anticoagulation
- Recurrent PE despite adequate therapy
- Massive PE with residual DVT in a patient at risk for further PE
- Propagation/progression of DVT during therapeutic anticoagulation
- Free-floating iliofemoral or IVC thrombus and severe cardiopul-monary disease and DVT
IVC filter placement has a prophylactic indication (i.e., in cases without current thromboembolic disease) in the following settings:
- Severe trauma without documented PE or DVT
- Closed head injury
- Spinal cord injury
- Multiple long-bone or pelvic fractures
- Patients at high risk (e.g., immobilised or in an intensive care unit)
NICE7
Patients with evidence of PE or IVC, iliac, or femoral-popliteal DVT and one or more of the following:
- Absolute or relative contraindication to anticoagulation
- Complication of anticoagulation
- Failure of anticoagulation
- Recurrent PE despite adequate therapy
References
* Cook-sponsored or Cook supported studies.
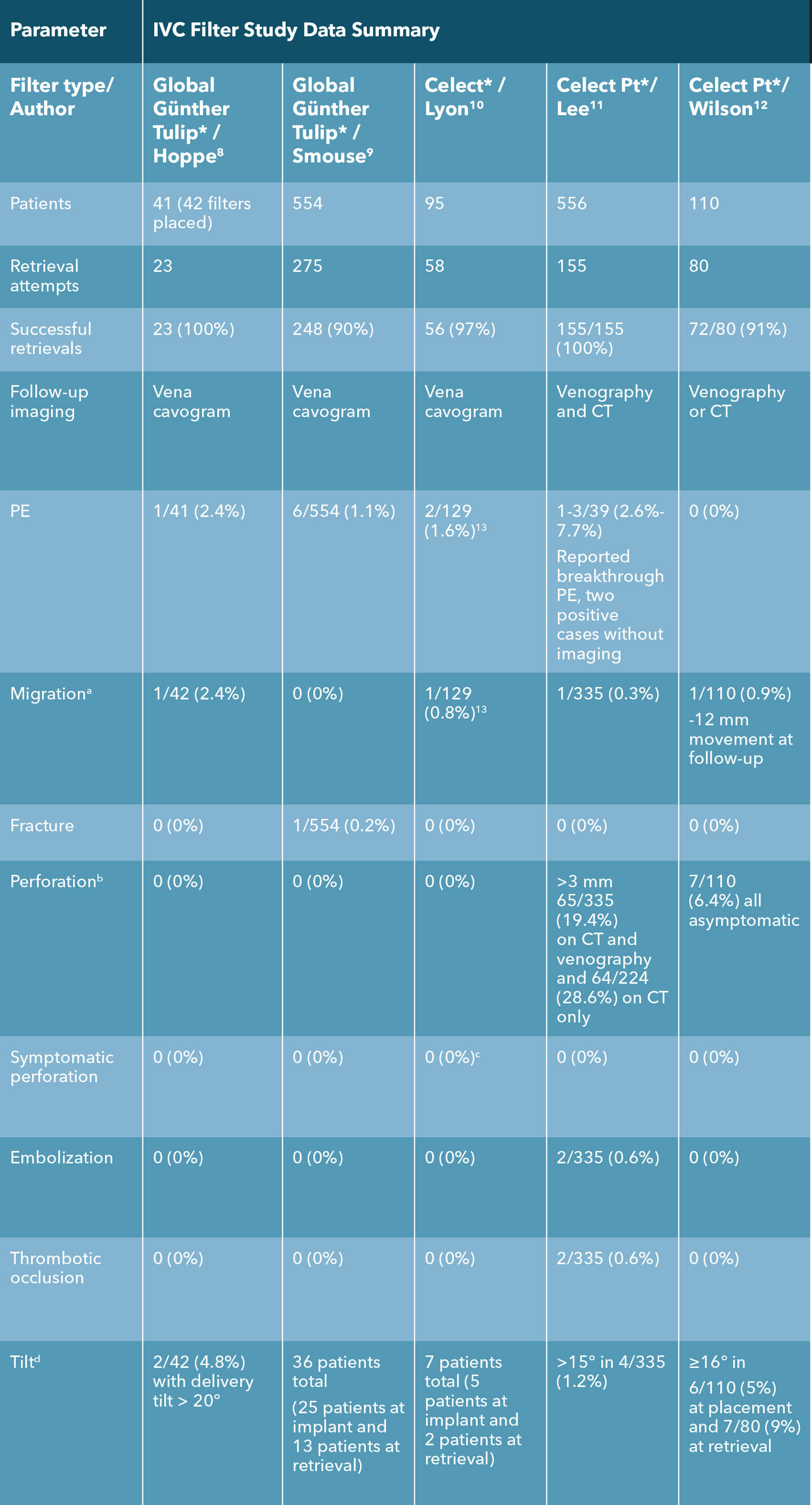
a Migration: Change of > 2 cm in filter position.
b Definitions varied. Günther global/Celect: extension out of the IVC wall; Denali/G2: extension 3 mm or more beyond the IVC wall; Option: extravascular penetration.
c At retrieval, 21 patients noted to have penetration or transmural incorporation. There was no extravasation, no hemorrhage, and no patient-reported symptoms associated with any of these filters that had incorporated into the IVC wall.
d Definitions varied slightly: Gunther/Celect: >16 degrees; Denali/Optease/G2/Option: >15 degrees.
Citations
- Evaluation of the Zilver Vena venous stent in the treatment of symptomatic iliofemoral venous outflow obstruction (VIVO clinical study) IDE #G110228; Global Clinical Study Number: 11-010; May 18, 2020.
- Zilver Vena Venous Self-Expanding Stent. Instructions for Use: IFU0091-8. Limerick, Ireland; 2021.
- Shamimi-Noori SM, Clark TWI. Venous stents: current status and future directions. Tech Vasc Interv Radiol. 2018;21(2):113-116.
- Summary of safety and effectiveness data (SSED): Zilver Vena Venous Self-Expanding Stent. Food and Drug Administration Web site. http://www.accessdata.fda.gov/cdrh_docs/pdf20/P200023B.pdf. Accessed September 8, 2021.
- CIRSE. Quality improvement guidelines for percutaneous inferior vena cava filter placement for the prevention of pulmonary embolism. 2009. https://www.cirse.org/wp-content/uploads/2018/11/2009_Percutaneous-Inferior-Vena-Cava-Filter-Placement-for-the-Prevention-of-Pulmonary-Embolism.pdf.
- ARC-SIR 2016 practice guideline for the performance of inferior vena cava (IVC) filter placment for the prevention of pulmonary embolism. 2016. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/IVC-FliterPlacement.pdf?la=en.
- NICE Pathways. Treating venous thromboembolism. 2016.
- Hoppe H, Nutting CW, Smouse HR, et al. Gunther Tulip filter retrievability multicenter study including CT follow-up: final report. J Vasc Interv Radiol. 2006;17(6):1017-1023.
- Smouse HB, Rosenthal D, Thuong VH, et al. Long-term retrieval success rate profile for the Gunther Tulip vena cava filter. J Vasc Interv Radiol. 2009;20(7):871-877; quiz 878.
- Lyon SM, Riojas GE, Uberoi R, et al. Short- and long-term retrievability of the Celect vena cava filter: results from a multi-institutional registry. J Vasc Interv Radiol. 2009;20(11):1441-1448.
- Lee BE, Van Allan RJ, Friedman ML, Lipshutz HG. Complications and retrieval characteristics of Celect Platinum inferior vena cava filters. J Vasc Surg Venous Lymphat Disord. 2018;6(2):163-172.
- Wilson I, Robinson G, McCann-Brown JA, Sherman SM, McWilliams R. Early experience of the Cook Celect Platinum inferior vena cava filter in the United Kingdom. Presented at: CIRSE 2019.
- Prospective Study of the Cook Celect Filter, Including Permanent and Retrievable Use (Protocol #05-507). 2009.