
This article is part of our Ask the CMO series, where Cook Medical’s chief medical officer, Dr. John Kaufman, answers questions. Learn more about Dr. Kaufman in his Meet Our Leaders bio.
Q: You have worked with many other medical device companies and many people in the industry, such as sales reps, trainers, product managers, and regulatory scientists. From a physician’s perspective, how did Cook differentiate itself?
Dr. Kaufman: The reason that I was so excited to join Cook is that for my entire career I always felt that anyone that I worked with from Cook is more of a partner with aligned interests toward doing the best thing for the patient than a transactional relationship. I’ve never felt with Cook that there was a pressure to use something that somebody needed to fill a quota for or convince me to switch to something. It was never that way. It is always with Cook that I felt aligned, to the extent that I could trust anyone from Cook to say, “We don’t have the thing you are looking for, but this other company does.” When I first then began investigating or meeting people as potentially becoming part of Cook that culture was very clear.
What is different about Cook is that, although that culture may be present at other companies, it is such a strong presence throughout all of Cook. There is a shared alignment of goals between those of us who are taking care of people and trying to do the best thing for the person in front of us regardless of other factors. Overall, that is how Cook operates.
Obviously, we are a company, we have to somehow make enough money to stay afloat and make enough money to take care of the people who work for us as well. So, decisions can’t always be purely altruistic. We do have to make some of those decisions, but that is always in the framework of is this really the right thing to do, not just for us but for the patients of physicians.
What is an area that could potentially be improved on?
What could we do better? I think everyone has a different answer based on their own perspective, and as Cook customer it would be great if we could get new or updated devices through the pipeline and into clinical practice as fast as possible. We have so much fantastic stuff in the wings and I want it now! On a serious note, we also need to focus on preserving our unique culture and relationships with physicians as we navigate the complex and highly regulated world that we now operate in. These two things have been drivers for Cook from the beginning and are in a sense timeless.

This article is part of our Ask the CMO series, where Cook Medical’s chief medical officer, Dr. John Kaufman, answers questions. Learn more about Dr. Kaufman in his Meet Our Leaders bio.
Q: How do you balance your connection with Cook with what is the best option or device for your patient?
Dr. Kaufman: I do love working for Cook. It is one of the best things that has ever happened to me professionally, if not the best thing. And I also love taking care of patients and I so appreciate the ability to do both things at the same time. It is not a thing I take for granted at all, but it is an incredible privilege.
The culture at Cook and the culture of medicine are very similar: You do what is best for the patient in front of you.
Don’t do what is best for you or what might be best for your friend or the company you work for or the hospital you are working for or the professional organization that you are a member of. You do what is right for the person in front of you and that guides you all the time.
That prevents really any issue from arising of, “Should I be using a Cook catheter or a non-Cook catheter?” Cook catheters are the best catheters — they just are. So, I don’t have any qualms about using what I think is the best device. There are other devices that other people do better than us and I will use them because I think it is the right thing. I’m glad that it is evident that I am excited to work for Cook as it is an awesome group of people and an awesome organization.

L-R: Rick Simms (Cook Medical, National Manager, GPO Account Executives), Amber Pastorek (Cook Medical, Account Executive Manager), Glenn Coleman (Premier, Chief Financial Officer & Chief Administrative Officer), Bob Stanley (Cook Medical, National GPO Account Executive), Brian Majoy (Cook Manager, Account Executive Manager), Chad Wissner (Cook Medial, Account Executive), Bruce Radcliff (Premier, President of Supply Chain Services) and Michael Alkire (Premier, President and CEO)
Bloomington, Ind. — Cook Medical is honored to receive a 2025 Legacy Supplier Award from Premier, Inc. This supplier award recognizes Cook’s deep connections and commitments to hospitals, supply chain leaders and other healthcare organizations.
Premier is a leading healthcare improvement and technology company that unites an alliance of more than 4,350 U.S. hospitals and health systems. Each year, Premier announces award winners at their Breakthroughs Conference. The awards in various categories go to organizations that demonstrate unwavering commitment to delivering top value products and services designed to continually improve patient care.
Cook Medical was honored to be among a select number of organizations that received the 2025 Legacy Supplier Award. Companies earn this award for long-standing support of Premier members through exceptional local customer service and engagement, value creation through clinical excellence and commitment to lower costs. Supplier Legacy Award winners have a tenure of more than three years as a Premier contracted supplier.
What separates Cook from other suppliers is our Customer Portal, which makes the ordering experience more convenient and efficient for customers. Cook also offers a supplier risk dashboard and a biweekly global supply chain report.
“For more than two decades, Cook Medical has been proud to collaborate with Premier,” said Bob Stanley, a national GPO account executive with Cook’s BusinessCare Integration team.”We are truly honored to receive this award from Premier. It reflects our shared mission and our dedication to delivering value to Premier’s membership. We’re excited about what lies ahead and continuing to grow with our customer’s evolving needs.”
To learn more about the advantages of partnering with Cook Medical, visit our BusinessCare Integration site.
About Premier, Inc.
Premier is a leading technology-driven healthcare improvement company, providing solutions to two-thirds of all healthcare providers in the U.S. Playing a critical role in the rapidly evolving healthcare industry, Premier unites providers, suppliers, payers and policymakers to make healthcare better with national scale, smarter with actionable intelligence and faster with novel technologies. With integrated data and analytics, collaboratives, supply chain solutions, consulting and other services, Premier enables better care and outcomes at a lower cost.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.

Some Cook Urology products that are included in the contract with Vizient
Bloomington, Ind. — Cook Medical has been awarded a contract from Vizient for our portfolio of urology devices. This agreement provides increased access to Cook’s urology products for Vizient clients while further strengthening Cook’s decades-long, physician-focused connections with hospitals across the country. Vizient is the nation’s largest provider-driven healthcare performance improvement company.
Through the contract, Vizient clients will experience streamlined ordering and delivery process and continue to receive excellent clinical and supply chain support from Cook Medical representatives. This agreement also allows physicians to continue treating patients with Cook products they know and trust at competitive price points and reliable product availability.
The urology products in this contract include:
“This contract is another milestone in our collaborative relationship,” said Rob Faulkner, senior director of global sales for Cook Medical’s MedSurg division. “We are excited to continue working with Vizient and making our comprehensive line of urological disposables more accessible to physicians and patients.”
In addition to this contract, Cook has previously earned recognition from Vizient. In 2023, Cook earned Vizient Supply Assurance Supplier of the Year Award. To learn more about the advantages of partnering with Cook Medical, visit our BusinessCare Integration site.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.
CAUTION – INVESTIGATIONAL DEVICE. Limited by United States law to investigational use.
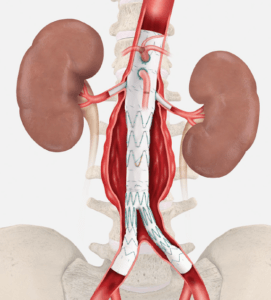
Cook Medical’s investigational ZFEN+ device
Bloomington, Ind. — Cook Medical has enrolled the final patient in the global clinical study of the ZENITH® FENESTRATED+ Endovascular Graft (ZFEN+). This milestone signifies the completion of patient recruitment in the pivotal study and demonstrates Cook’s commitment to innovating medical devices for the treatment of aortic diseases.
The investigational ZFEN+ clinical study is being conducted under an Investigational Device Exemption (IDE) approved by the U.S. Food and Drug Administration (FDA) and with authorization for a clinical investigation under Medicines and Healthcare products Regulatory Agency (MHRA). This clinical study will assess the safety and effectiveness of the ZFEN+ used in combination with the investigational Zenith® Universal Distal Body 2.0 Graft (Unibody2), the investigational Bentley BeGraft Balloon-Expandable FEVAR Bridging Stent Graft System (BeGraft) and the commercially available Zenith® Spiral-Z® AAA Iliac Leg Graft (ZSLE).
“Now that enrollment is complete, we are one step closer to understanding the outcomes of patients treated with ZFEN+ and, ultimately, to advancing care for patients with complex abdominal and thoracoabdominal aneurysms,” said Johnny LeBlanc, director, product management, Aortic at Cook Medical. “Reaching this milestone is a direct reflection of the dedication shown by our investigator partners, study coordinators, and—most importantly—the patients who supported the study with their time and trust. We look forward to analyzing the data and sharing the results with the clinical community.”
The ZFEN+ is predicated on the commercially available Zenith Fenestrated (ZFEN) AAA Endovascular Graft but extends the proximal margin of aneurysmal disease that can be treated endovascularly to include patients with more complex aortic disease involving one or more of the major visceral arteries. The ZFEN+ is an endovascular graft which includes up to 5 precisely located fenestrations, or a combination not to exceed a total of 5 made up of fenestrations and 1 scallop (cut-outs from the proximal margin of the endograft material) to accommodate visceral vessels. Physicians can order fenestrations and scallops specifically to match the patient’s unique anatomy. Overall, the ZFEN+ allows for the endovascular treatment of patients with aortic aneurysms and maximizes the seal zone to exclude the aneurysm. The product was granted Breakthrough Designation from the FDA in 2021.
“Reaching full enrollment in this important clinical study is a major milestone for Bentley and a significant step toward making our BeGraft Bridging stent available to patients in the United States. It underscores the strength of our collaboration with Cook Medical and reflects our shared commitment to advancing innovative vascular solutions that improve patient outcomes,” said Sebastian Büchert, Bentley CEO.
“Amazing work from the clinical research team on the enrollment of the final ZFEN+ patient. The clock is now ticking for us to have a patient specific solution for complex aortic aneurysms that will optimize seal without compromise,” said Dr. Gustavo Oderich, the global principal investigator of the clinical study.
Additional information about the clinical study will be available at www.clinicaltrials.gov.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.
CAUTION: Investigational Device. Limited by United States law to investigational use.
Cook Medical’s investigational Advance Evero™ 18 Everolimus-Coated PTA Balloon Catheter (Evero DCB)
Bloomington, Ind. — The U.S. Food and Drug Administration (FDA) has granted approval for Cook Medical to initiate an Investigational Device Exemption (IDE) study on the Advance Evero™ 18 Everolimus-Coated PTA Balloon Catheter (Evero DCB).
The clinical study will assess the safety and effectiveness of Evero DCB when compared to commercially available paclitaxel-coated balloons for the treatment of peripheral artery disease (PAD). Currently there are no everolimus-coated balloon devices commercially available in the US, and this IDE study is the first U.S. head-to-head evaluation of everolimus- and paclitaxel-coated balloons to treat lesions of the superficial femoral and popliteal arteries.
The EVERO Trial is a prospective, U.S. multi-center, stratified, blinded, randomized control trial with a parallel pharmacokinetic (PK) study. Cook intends to enroll 410 patients in the pivotal trial and 30 patients in the PK evaluation. The primary safety endpoint is a composite of freedom from device- or procedure-related death at 30 days, freedom from target limb major amputation at 12 months, and from target lesion revascularization (TLR) at 12 months. The primary effectiveness endpoint is primary patency, defined as peak systolic velocity ratio (PSVR) ≤2.4 at 12 months and freedom from clinically driven TLR (CD-TLR) at 12 months.
“Advance Evero 18 leverages our deep understanding and history of innovation in drug elution therapies for peripheral arterial disease,” said Dr. John Kaufman, Cook Medical’s chief medical officer. “The head-to-head study design will provide the answers that physicians and patients want. I’m confident that Advance Evero will be an excellent complement to our dedicated PAD portfolio aimed at improving long-term clinical outcomes for these patients.”
Additional information about the clinical study will be available at www.clinicaltrials.gov. You can also learn more about Cook’s portfolio of peripheral intervention products.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.
News coverage of this announcement
Endovascular Today: Cook’s Advance Evero 18 Everolimus-Coated Balloon IDE Study Plan Approved by FDA
Vascular News: FDA grants approval for IDE study on Advance Evero 18 everolimus-coated balloon catheter
 We are passionate about innovation in the supply chain industry. Our goal is to connect with and listen to healthcare professionals to identify unmet needs and use our expertise to imagine new solutions. For years, we have consistently heard that there is a decreasing amount of emerging talent in our industry and that there is a clear need to foster new members. That is why we created the Supply Chain Fellowship program.
We are passionate about innovation in the supply chain industry. Our goal is to connect with and listen to healthcare professionals to identify unmet needs and use our expertise to imagine new solutions. For years, we have consistently heard that there is a decreasing amount of emerging talent in our industry and that there is a clear need to foster new members. That is why we created the Supply Chain Fellowship program.
How it works
Our fellowship program is the first of its kind. One of the most important aspects is finding a fellow new to the industry with no preconceived ideas, and without ties to either organization. We then partner with a healthcare provider, as the fellow can move freely between the two organizations. This allows the fellow to see patterns and connections that benefit both ends of the supply chain and create a tangible output.
This Cook-funded Fellowship provides a recent MBA graduate with the opportunity to work as a dedicated Fellow, between Cook and the provider organization, to complete a project or group of projects. Selected projects should identify scalable methods to improve supply chain efficiency, visibility, resiliency, and alignment, with the possibility of implementation during the Fellowship. The Fellow will publish a white paper about the project(s) and experience, and present findings at industry conferences. Upon completion, the goal is for the Fellow to gain employment at a healthcare provider organization.
The goal of each fellowship is to bring suppliers and providers closer together. Both organizations agree on a specific project in advance of finding a fellow who is best suited with the appropriate skills to problem solve. Fellows are responsible for finding scalable, real-world solutions and to share those solutions with the industry by creating a white paper. This is a way for their experience to continue to live on and foster innovation within the healthcare supply chain.
Below is a video showcasing each of our fellows and their experiences. Watch the video to learn more!
6375710639112
brightcove
true
Fellowship recipients
The recipients of the Cook Medical Supply Chain Fellowship have had—and continue to have—amazing careers!
Resa Wise, 2023-2024 recipient
 Resa Wise received the Cook Medical Supply Chain Fellowship in 2023. Resa Wise was Cook’s—and the medical device industry’s—very first Supply Chain Fellow. This program is a collaboration between Cook and BJC, a health system in St. Louis. Resa holds a BS in Operations Management and earned her MBA from Utah State University.
Resa Wise received the Cook Medical Supply Chain Fellowship in 2023. Resa Wise was Cook’s—and the medical device industry’s—very first Supply Chain Fellow. This program is a collaboration between Cook and BJC, a health system in St. Louis. Resa holds a BS in Operations Management and earned her MBA from Utah State University.
In addition to her education, Resa has prior supply chain experience in grocery retail. She’s also worked in a lab that manufactured satellites, which helped prepare her for the tight regulations in medicine.
In her free time, Resa likes to hike and camp in the Idaho and Utah mountains where she lives. She is now a Deployment Planner for ICU Medical.
Rajeev Rohira, 2024-2025 recipient
 Rajeev Rohira, the 2024-2025 Supply Chain fellow, is a recent graduate of the University of Tennessee’s MBA program. He holds extensive experience in data analytics and automation due to past roles and internships. Although he possesses a vast array of analytical and technical skills, Rajeev Rohira sought to improve his knowledge on supply chain operations and processes. As such, he spent his fellowship working with individuals from a variety of positions ranging from sales to operations. By holistically exploring the ins and outs of both the supplier and the provider, he constructed his solutions with precise and updated knowledge of how the supply chains work on both ends. Starting in September 2025, Rajeev will be a consultant with a consulting firm called SLKone, where he will have the opportunity to continue to apply his skills to the healthcare industry.
Rajeev Rohira, the 2024-2025 Supply Chain fellow, is a recent graduate of the University of Tennessee’s MBA program. He holds extensive experience in data analytics and automation due to past roles and internships. Although he possesses a vast array of analytical and technical skills, Rajeev Rohira sought to improve his knowledge on supply chain operations and processes. As such, he spent his fellowship working with individuals from a variety of positions ranging from sales to operations. By holistically exploring the ins and outs of both the supplier and the provider, he constructed his solutions with precise and updated knowledge of how the supply chains work on both ends. Starting in September 2025, Rajeev will be a consultant with a consulting firm called SLKone, where he will have the opportunity to continue to apply his skills to the healthcare industry.
Emma Strieter, current fellow

Bio: A recent M.B.A. graduate, Em seeks to apply her unique background in both biology and supply chain to improve efficiency and transparency within the healthcare industry. She, originally from Michigan, enjoys reading and spending time outdoors with her pup.
School: University of South Carolina; University of Tennessee-Knoxville
Area of study: B.S. Biological Sciences, B.S. Spanish; M.B.A. Supply Chain
Goal of fellowsip project: To partner with Mayo Clinic in evaluating the benefits and challenges associated with data sharing between suppliers and providers, highlighting use of the Surgence platform.
Paul Vang, current fellow
 Bio: A life-long Wisconsinite and recent M.B.A. graduate specializing in supply chain management, Paul is focused on enhancing healthcare logistics to drive better patient outcomes and elevate customer satisfaction. In his free time, he enjoys training martial arts, watching documentaries, and trying new foods during his travels.
Bio: A life-long Wisconsinite and recent M.B.A. graduate specializing in supply chain management, Paul is focused on enhancing healthcare logistics to drive better patient outcomes and elevate customer satisfaction. In his free time, he enjoys training martial arts, watching documentaries, and trying new foods during his travels.
School: University of Wisconsin-Madison
Area of Study: B.S. Chemistry, Secondary Education; M.B.A., Supply Chain Management
Goal of fellowship project: In collaboration with Indiana University (IU) Health’s Operational Excellence team, the fellow will identify and assess opportunities for process improvement and efficiency across IU Health’s supply chain. The project will culminate in a set of actionable solutions supported by a detailed project plan and implementation timeline.
Published white papers
Each fellow creates a published white paper accessible to anyone. This is our way of ensuring the information can support future innovation for other suppliers and providers who may want to pursue similar solutions as to what our fellows have created.
2023: BJC Healthcare & Resa Wise
- Goal of creating an end-to-end value stream map to identify gaps, efficiencies, and cost-saving opportunities.
2024: Cleveland Clinic & Rajeev Rohira
- Goal of formulating a new demand planning approach between the two organizations as well as understanding the providers’ order behavior.
Let’s connect
Let us know if you are interested in working together on a future partnership through our fellowship program! Reach out to us at SCS-AMER@CookMedical.com, or you may also use this Calendly link to schedule time to speak to one of our supply chain solution managers to learn more about this program.

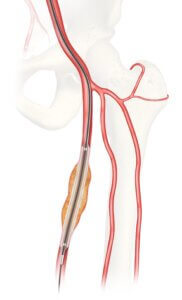
Cook Medical’s Zenith® Iliac Branch Device (ZBIS)
Bloomington, Ind. — Cook Medical’s Zenith® Iliac Branch Device (ZBIS) is now commercially available in the United States with FDA approval as an endovascular treatment option for aortoiliac or iliac aneurysmal disease. Cook is excited to bring this advanced product to the US and improve the lives of patients with aortoiliac or iliac aneurysms.
Cook is also excited to announce several of the first implants of ZBIS in the United States. The physicians who performed the first implants include:
- Andres Fajardo, IU Health Methodist Hospital
- James Black III, Johns Hopkins Hospital
- Karan Garg, NYU Langone Medical Center
- Jason Lee, Stanford Hospital
- Caitlin Hicks, Johns Hopkins Hospital
“This product offers an endovascular treatment option for many patients who do not meet the criteria of other commercially available technology. The ZBIS anatomical inclusion criteria is broader and is backed by 5-year pivotal data. It’s a great addition to Cook’s portfolio of aortic devices that addresses an important patient need,” said Johnny LeBlanc, director of Cook Medical’s Aortic specialty.
About ZBIS
When patients suffer from aortoiliac or iliac aneurysms, they risk clots and ruptured aneurysms. The ZBIS graft treats aortoiliac or iliac aneurysms and provides patients an improved quality of life through 5 years.
The ZBIS graft treats aortoiliac or iliac aneurysms while maintaining blood flow to the internal iliac artery. Built on the proven Zenith platform, ZBIS expands Cook’s current aortic portfolio to include treatments for iliac and aortoiliac aneurysms. This graft offers benefits such as:
- Preserving a patient’s quality of life by preserving internal iliac function and reducing the incidence of buttock claudication, impotence, and other ischemic complications. 1
- Delivering a durable aneurysm repair with long-term data to support 0% iliac artery aneurysm enlargement on the ZBIS side. 1
- Treating a wide range of patients by having a smaller device proximal diameter of 12mm and offering two common iliac segments lengths (45 mm and 61 mm).
FDA approved the Zenith Iliac Branch on 30 May 2025. The Zenith Iliac Branch, when used with the necessary additional components (Zenith AAA and a covered bridging stent), is indicated for the endovascular treatment of patients with an aortoiliac or iliac aneurysm to preserve internal iliac arterial blood flow when the distal sealing site in the common iliac artery is insufficient for the AAA device alone and when the vessel morphology is suitable for repair.
To learn more about ZBIS, you can visit the product page here.
About Cook Medical
At Cook Medical, we are passionate about making unique, quality medical devices and connecting with people to improve lives. Founded on inventing, manufacturing, and delivering medical devices, we provide healthcare professionals with the tools they need to help their patients return to living.
Our commitment to innovation involves bringing new products to market and keeping existing products relevant to a changing healthcare landscape. We believe in using our business to help people and communities thrive by creating inclusive, supportive, and healthy environments.
We are proud of our history of innovative firsts and the impact we have on patients and communities. With headquarters in Bloomington, Indiana, and manufacturing facilities and offices in various global locations, we challenge ourselves to maintain a global perspective while focusing on local impact.
Follow Cook Medical at CookMedical.com and on LinkedIn.
1 US Pivotal Clinical Trial Data PMA (P020018/S064)
A total of 40 patients were treated with ZBIS and the iCast covered stent between 01 April 2014 and 06 May 2015. Study patients were enrolled across 18 investigational sites in the United States.













