Proven
Long-term results delivered
Zilver PTX offers a less-invasive option for patients with life-limiting conditions associated with PAD, providing long-term clinical results compared to standard endovascular treatments1. When compared to the competition, Zilver PTX provides:
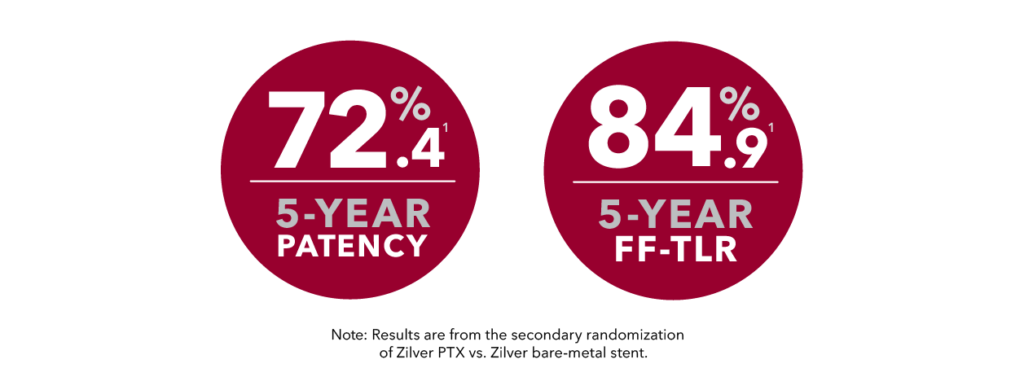
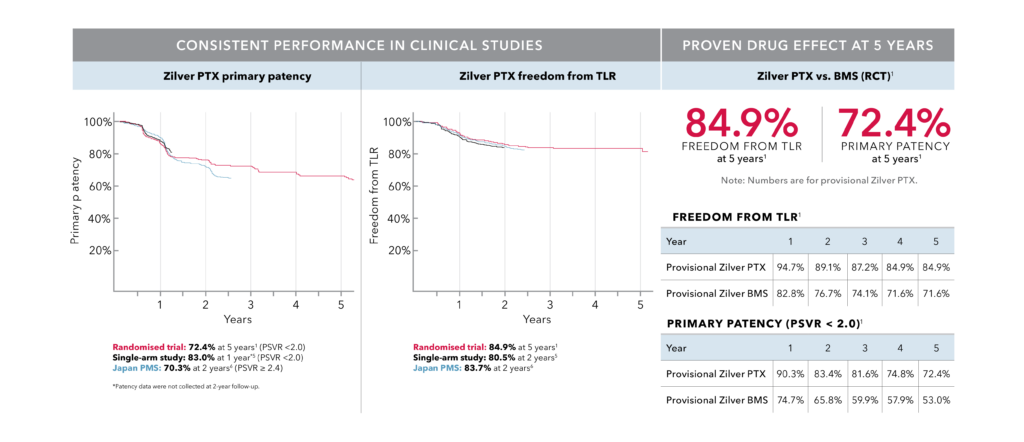
- A reduced risk of restenosis and reinterventions with superior long-term outcomes (5 years) to standard of care (BMS)1
- Similar primary patency, FF-TLR, amputation, and survival rates at 5-years compared to bypass while also having significantly shorter hospital stays at procedure and 30-day complications2
- Similar primary patency and CD-TLR rates in both real-world (1-year)3 and RCT (5-year)4 when compared to Eluvia without the concerns of permanent polymers
Proven at 5 years | Zilver PTX RCT
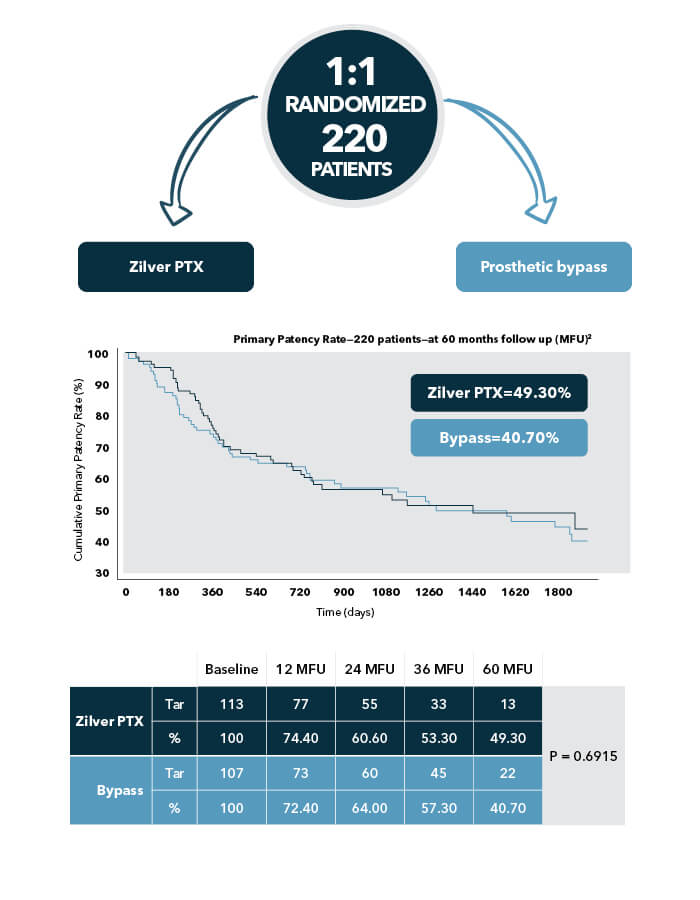
Proven against bypass | ZILVERPASS 5-year results
The ZILVERPASS objective was to evaluate the performance of Zilver PTX compared to prosthetic bypass surgery for the treatment of femoropopliteal TASC C & D lesions. The RCT included an economic analysis at 36 months and a final clinical publication at 60 months.
A bold decision: The Zilver PTX randomized controlled trial was designed from the start to include 5-year follow-up data that would determine not only long-term benefits but also long-term safety signals or adverse events.
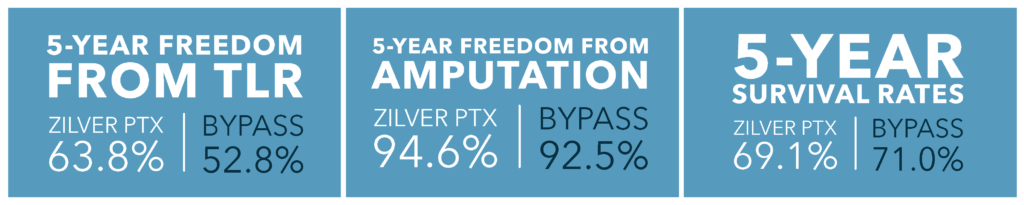
Compared to patients who underwent prosthetic bypass surgery, Zilver PTX patients had:
• Significantly shorter hospital stays at procedure7
• Significantly fewer complications through 30 days7
• Similar survival rate through 5 years2
Economic analysis (US)
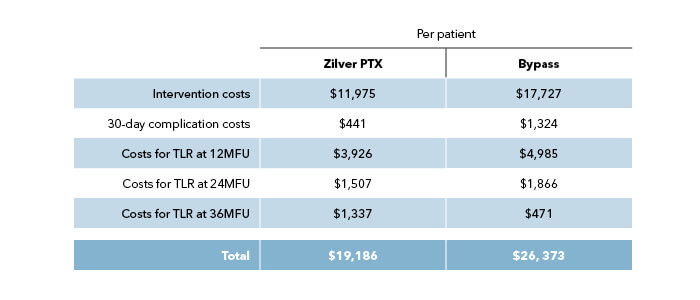 The economic analysis, taking into account procedural, hospitalization, and reintervention costs, showed a clear cost-benefit for Zilver PTX, in the USA reimbursement model.8
The economic analysis, taking into account procedural, hospitalization, and reintervention costs, showed a clear cost-benefit for Zilver PTX, in the USA reimbursement model.8
Conclusion: These final 5-year results unequivocally establish the non-inferiority of the Zilver PTX stent compared to above-the-knee prosthetic bypass surgery, underscoring their comparable effectiveness and safety profiles.2
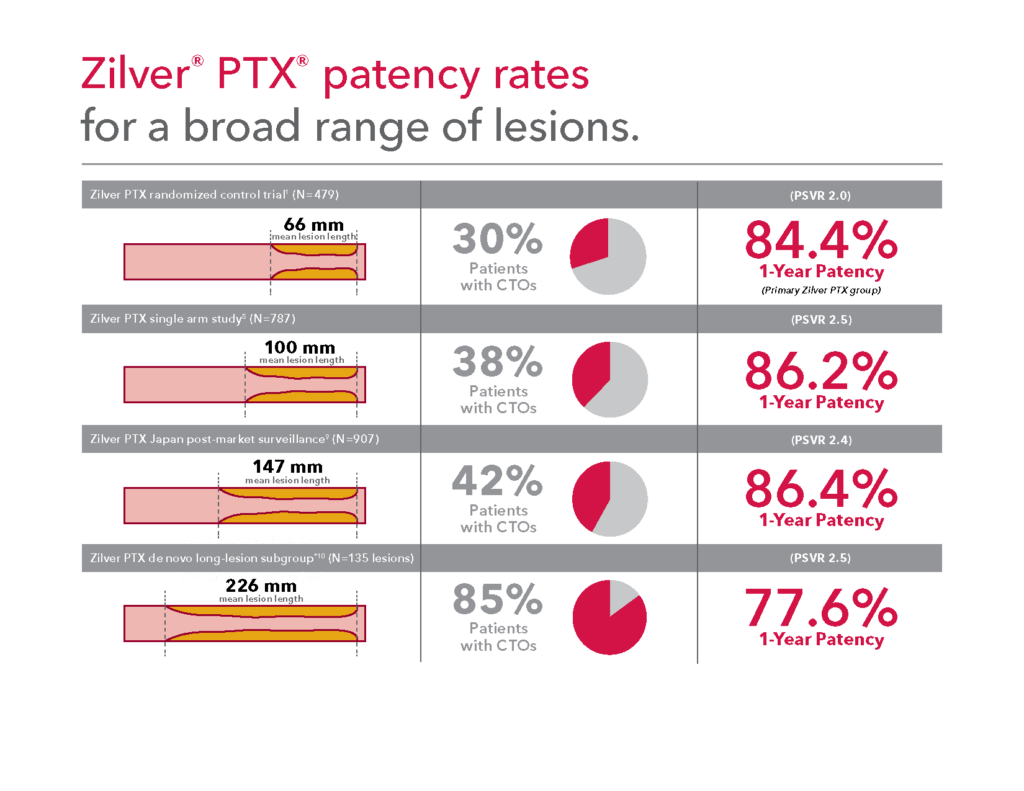
Proven in a broad range of lesions
*Subgroup of patients for the Zilver PTX single arm study. Long lesion group was 135 lesions that were > 15 cm, corresponding to TASC C or D. Results from a non-pre-specified post-hoc analysis.
Note: CTO = Chronic Total Occlusion; PSVR = Peak Systolic Velocity Ratio.