Innovative
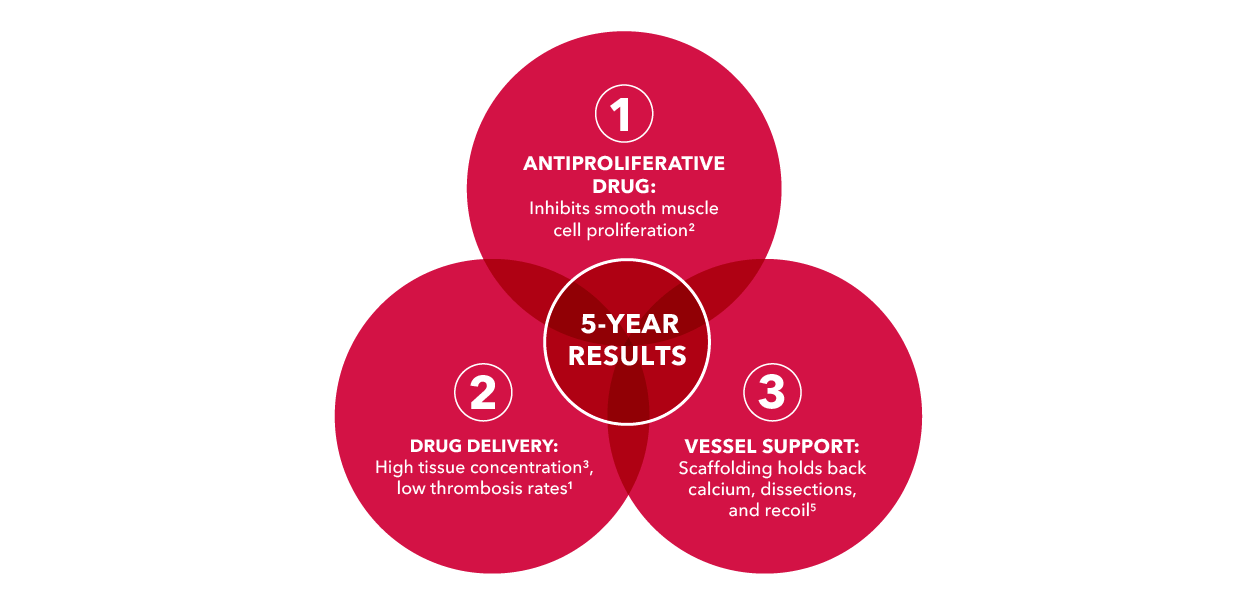
Three essentials for achieving 5-year results
Only Zilver PTX combines an antiproliferative drug with, polymer-free drug delivery and vessel support to demonstrate superior 5-year results against PTA and Zilver bare-metal stents.1
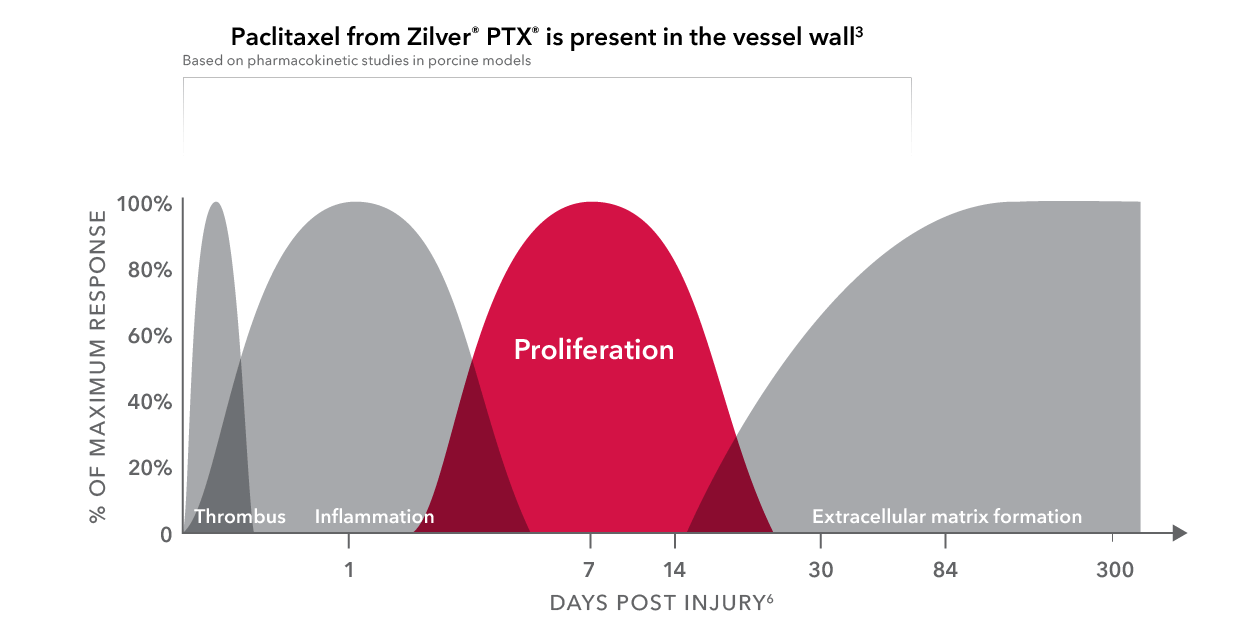
When drug elution is most needed
Short-term paclitaxel delivery in the SFA has been proven beneficial.
How drug elution works
Zilver PTX is the only polymer-free SFA drug-eluting stent. Our proprietary, polymer-free coating process is shown to be safe and effective while eliminating the potential risks associated with permanent polymers.
Release
More than 98% of the paclitaxel coating is released from the stent within 72 hours.*3
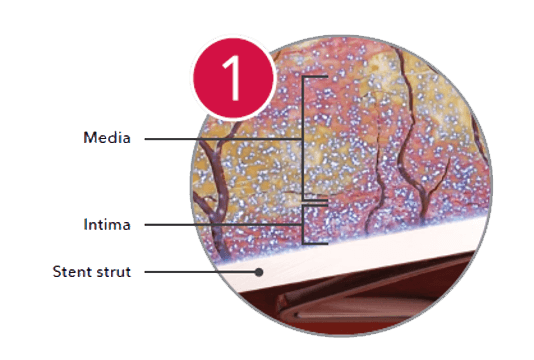
Absorption
Paclitaxel remains in the artery through 56 days.*
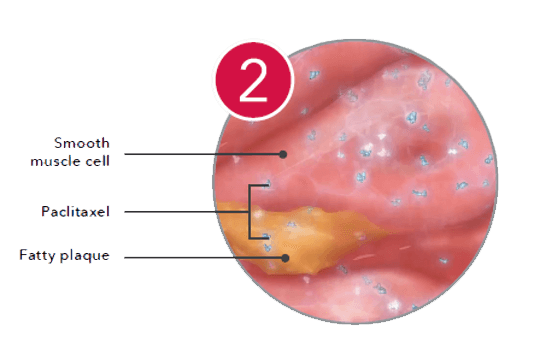
Inhibiting
Inside the cell, the drug binds to microtubules and inhibits mitosis.*
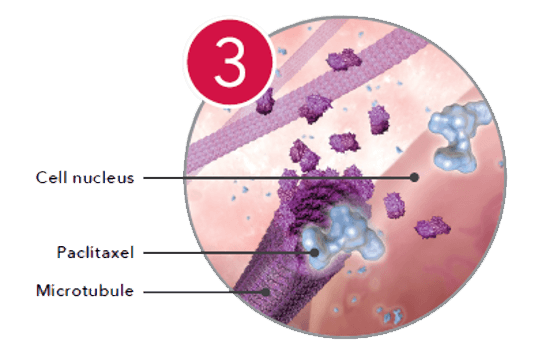
*Based on pharmokinetic studies in porcine models
Broad offering
Treat a wider range of patients and lesions
Zilver PTX will enable you to standardize your drug-elution program and reduce the number of suppliers on the shelf. Zilver PTX offers more size configurations and indications for longer lesion lengths than Eluvia, enabling you to treat more patients with Zilver PTX.
· Available in 5, 6, 7, and 8 mm diameters
· Available in lengths up to 140 mm
· Approved for lesion lengths up to 300 mm
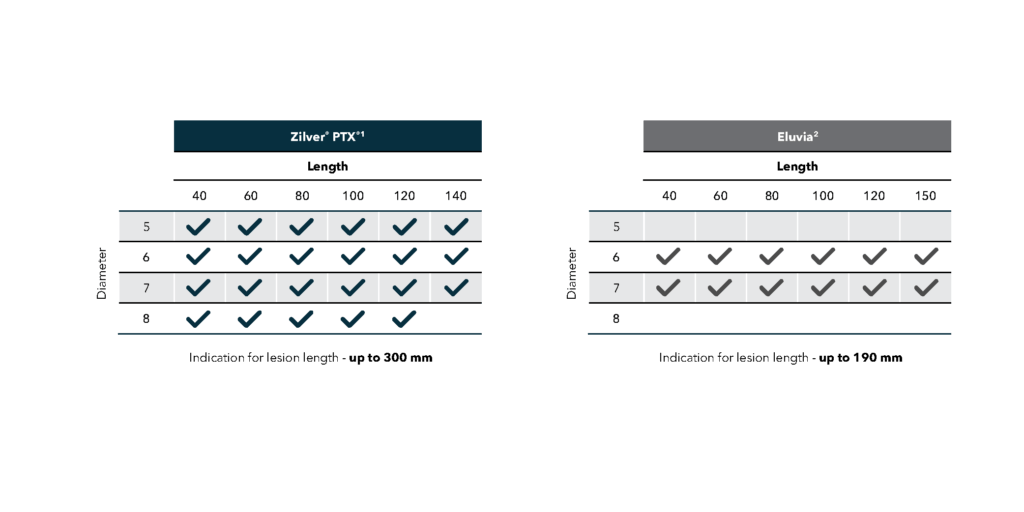 *Size availability as of June 2025
*Size availability as of June 2025
ELUVIA is a registered trademark of Boston Scientific Corporation or its affiliates. Learn more here.